Jamie B. Rosenberg, MD; Lewis A. Eisen, MD
Crit Care Med. 2008;36(12):3151-3155.
©2008 Lippincott Williams & Wilkins
Posted 12/12/2008
Medical and nursing staff in the intensive care unit (ICU) concentrate the majority of their efforts on problems seen as immediately life-threatening. This may lead to lack of attention to other serious issues.
Mechanically ventilated patients in the ICU are at risk for exposure keratopathy. This condition predisposes to microbial keratitis, which may lead to corneal perforation and visual loss. Although attention has been focused on prevention of nosocomial infections, such as catheter-associated bloodstream infections, healthcare-associated pneumonia, urinary tract infections, and surgical site infections, comparatively little attention has been focused on prevention of microbial keratitis.[1]
In observational studies, 20% to 42% of patients in ICU develop exposure keratopathy.[2-4] Protocols have been developed to prevent corneal damage from exposure, but there is no widely accepted standard of care. Standard critical care textbooks aimed at physicians and nurses pay little attention to eye care in the ICU. The purpose of this article was to review the epidemiology, pathophysiology, and clinical presentation of exposure keratopathy and microbial keratitis. Additionally, we review screening and prevention of these conditions with a meta-analysis of two of the best-studied methods: moisture chambers (e.g., polyethylene film) and lubricating ointments.
Definitions
Definitions adapted from MedlinePlus:[5]
Lagophthalmos—pathologic incomplete closure of the eyelids (Fig. 1).
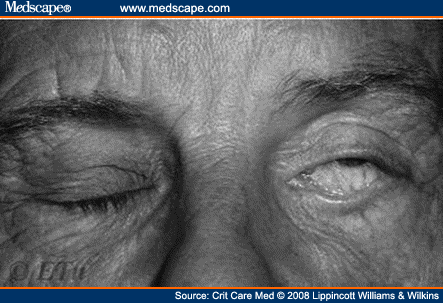
Figure 1.Lagophthalmos from http://eyetext.net
Keratitis—inflammation of the cornea
Keratopathy—a noninflammatory disease of the cornea
Chemosis—conjunctival swelling
Scleritis—inflammation of the sclera (white outer coat enclosing the eyeball)
Endophthalmitis—inflammation affecting the interior of the eyeball
Tarsorrhaphy—the operation of suturing the eyelids together entirely or in part.
Epidemiology
Patients in the ICU are at increased risk of corneal dryness and ulceration if they are unable to close their eyes. Many patients in the ICU who require intubation are sedated and/or paralyzed. Mercieca et al. found that 75% of such patients have lagophthalmos, predisposing them to corneal dryness. In addition, other critically ill patients are unconscious, predisposing to lagophthalmos even without pharmacologic sedation.[4]
In the same way that intact skin protects the body from cellulitis, an intact corneal epithelium protects the eye from infection. When the eye becomes dry, the patient develops small defects in the epithelium that can be seen with the slit lamp called superficial keratopathy or punctate epithelial erosions. The cornea becomes more permeable as the density and area of superficial keratopathy increases.[6,7] In a study by Imanaka et al.,[3] superficial keratopathy was present in 60% of intubated and sedated patients. In another study designed to evaluate the effect of lid closure on the cornea, McHugh et al.[8] found that 70% of patients with incomplete lid closure developed keratopathy compared with 28.9% of patients whose lids were completely closed.
Without an intact epithelium, the patient is more susceptible to microbial keratitis (Fig. 2). The true prevalence of microbial keratitis is unknown, but clusters of cases have been reported in the literature. Microbial keratitis can lead to complications including acute perforation, scleritis, and endophthalmitis, causing rapid visual loss.[9]
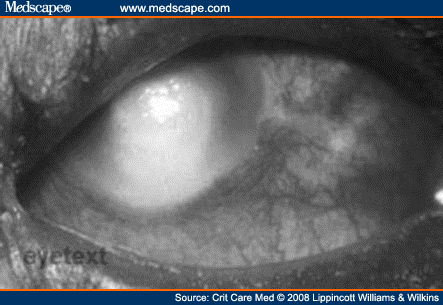
Figure 2.Microbial keratitis from http://eyetext.net
In severe cases of end-stage corneal infections refractory to maximal medical therapy, treatment of microbial keratitis may require corneal transplantation.[10] Unfortunately, survival rates of grafts performed for this indication tend to be lower than overall graft survival rates. In a large retrospective study, Sit et al.[11] found a 42.0% 5-yr graft survival for those transplants performed for corneal ulcers compared with a 64.5% survival for all other indications.
Pathophysiology
An intact ocular surface is a bulwark against infection. Few bacteria can overcome intact epithelium; most must rely on a break in the epithelial barrier.[12] The cornea is made of avascular, stratified, nonkeratinized epithelium that relies on tears for nutrients.[13] Tears lubricate the ocular surface, providing oxygen to the cornea and washing away noxious stimuli and potential pathogens. The corneal and conjunctival epithelia produce mucins that hold tears onto the eyes.[14] In addition, tears have bactericidal properties; proteins contained in tears, including lysozyme, lactoferrin, tear lipocalin, and secretory IgA help prevent infection.[12,15] During blinking, the palpebral conjunctiva glides over the cornea, spreading the tears evenly over the surface of the eye and preventing evaporation.[12] The process of tear evaporation changes the temperature of the conjunctival sac, making it unfavorable for bacterial growth.[16]
While asleep, lid closure protects the cornea by keeping it moist. Eyelid closure is an active process that requires contraction of orbicularis oculi and inhibition of levator palpebrae superioris.[17] Heavy sedation or use of paralytics inhibit these processes.[3,4] In addition, critically ill patients often have fluid imbalance and increased vascular permeability, which can cause conjunctival edema that hinders eye closure.[3] Positive pressure ventilation also causes conjunctival edema by raising the patient’s venous pressure and reducing drainage of blood from the ocular tissue.[3] This chemosis has been termed ventilator eye. When the lids are not completely closed, corneal dryness can develop.[4]
Numerous other factors may increase the risk of corneal exposure and resulting microbial infections in patients in the ICU. The high flow of oxygen through a facemask or nebulizers may lead to epithelial damage.[18] Tracheal suctioning may lead to aerosolization of respiratory pathogens onto the corneal epithelium.[16,19-21] In fact, suctioning may directly infect the cornea if the catheter is withdrawn over the eye, as occurs when nurses are positioned at the head of the bed for suctioning.[21] Hilton[19] showed that the left eye is more commonly infected; he speculated that this trend may be because right-handed nurses tend to withdraw the suction catheter directly over the left eye. Reduced rapid eye movement sleep in patients in the ICU may also increase rates of corneal exposure.[18] When comparing patients in the ICU, those with more significant medical illness are more susceptible to corneal damage: predictors of keratopathy are low score on the Glasgow Coma Scale, ICU stay longer than 1 wk, and significant metabolic derangement.[2]
It can be challenging to evaluate and appropriately treat the eyes of critically ill patients. A small degree of lagophthalmos, which can be significant in terms of maintaining corneal integrity, can easily be missed,[22-24] especially medially.[2] Ointments and eye drops used in an effort to protect the eyes can inadvertently spread infection when the same tube or applicator is used for both eyes.[2] It can also be difficult for doctors and nurses not trained in examining eyes to determine whether there is a contact lens in situ. If left in place for an extended period of time, contact lenses greatly increase the risk of corneal dryness and infection.[25]
As a network of nerves runs between the epithelial cells of the corneal surface, corneal damage causes severe pain.[14] Many critically ill patients cannot express the fact that their eyes hurt and need to be protected, resulting in a delay in attention to the eyes until damage is severe.
Once a patient is awake and alert, exposure keratopathy undergoes spontaneous resolution in the majority of cases. However, it can potentially cause corneal scarring and vision loss.[2] Although the incidence is unknown, case reports and series in the literature have documented incidences of severe infection causing visual loss sometimes requiring emergency corneal transplant.[19-21,26] Ideally, the cornea should be protected before it becomes damaged.
Clinical Presentations
Damage to the corneal epithelium can range from subtle to readily apparent. With a portable slit lamp, patients can be examined for superficial morphologic changes. Punctate epithelial keratopathy, also called microepithelial defects, are pinpoint epithelial elevations or slightly depressed erosions in the cornea. Macroepithelial defects are larger confluent areas of epithelial loss, commonly known as corneal abrasions.[2] Use of a penlight with a blue filter after instillation of 2% fluorescein dye is a useful bedside test for detecting corneal abrasions or ulcers. The dye outlines defects of the corneal and conjunctival epithelium.[27]
Once an infection develops, lid swelling, conjunctival swelling with hyperemia, and discharge or crusting of the lid margins can be the first signs in an ICU patient.[12] When examined with a slit lamp, bacterial corneal ulcers typically show a sharp epithelial demarcation with underlying dense suppurative stromal inflammation that has indistinct edges and is surrounded by stromal edema.[12] With a penlight exam, the method that is most often used by the staff in the ICU, the corneal epithelium will appear ulcerated, with a gray or white infiltrate under the epithelial defect.[28] Evidence of keratopathy, and especially any opacity or haziness of the cornea, should prompt consultation with an ophthalmologist.[29]
Screening and Prevention
To prevent bacterial keratitis, the critical care team needs to be aware of the dangers of exposure keratopathy. A study by McHugh et al. showed that when medical doctors in the ICU are examining patients for exposure keratopathy, they are able to detect it. In this study, twice each week, two junior ICU doctors examined lid position and ocular surface of all patients continuously sedated for >24 hrs, using a pen light with a blue filter. An ophthalmologist performed similar examinations with a slit lamp. A total of 48 examinations were done in 18 patients. ICU doctors had 77.8% sensitivity and 96.7% specificity in detecting keratopathy. All cases missed had erosions of <5% of the corneal surface.[8]
Studies have examined whether algorithms help to decrease the rate of corneal infections. Suresh et al. studied a population of unconscious or sedated patients. Patients with closed lids received no treatment; patients with exposed conjunctiva received lubricants; those with exposed cornea had lid taping and lubricants; and those patients who were ventilated in the prone position also received lid taping and lubricants. A total of 34 patients were entered into the study, but four patients were excluded from the study because eye care protocol was not followed appropriately. In addition, seven patients were excluded because the nurse made the wrong initial assessment of lid position. In the 23 patients for whom the algorithm was appropriately followed, there was an 8.7% rate of ocular surface disease, compared with a 42% prevalence in historical controls.[4,30] This algorithm was successful in lowering the rates of surface disease; however, the fact that the algorithm was not followed in all patients may limit the generalizability of the results.
Another guideline, designed by Parkin, was specifically focused on decreasing the risk of infection with Pseudomonas aeruginosa, a particularly virulent organism that causes a devastating and rapid keratitis. In this protocol, unconscious patients had eye care every 2 hrs. At the time of eye care, the eyes were inspected for lid swelling, conjunctival hyperemia, corneal clouding, and epithelial loss. If the corneas were exposed, lubricant was applied every 2 hrs. Those patients at risk for corneal exposure had their eyes taped shut. All tracheal suctioning was done from the side of the bed with the patient’s eyes covered. Daily swabs of the eyes were taken if respiratory cultures grow P. aeruginosa. Finally, if P. aeruginosa was grown from the eye swabs, topical gentamicin was started and ophthalmology consultation was requested. When these guidelines were adopted by the ICU, the conjunctival Pseudomonas isolation rate decreased significantly from 0.8% to 0.05% (p < 0.001), even though the frequency of isolation of Pseudomonas from the respiratory tract remained relatively high at 3.8%.[17]
Even when an attempt is made to follow an algorithm for eye care, the reality is that the algorithm is not always followed. Dawson audited an ICU that was following an algorithm for eye care. Only 25.5% of the patients had their eye assessment, and only 55.3% of the patients had eye care documented.[31] Even experienced nurses were found not to follow eye care protocols.[32] A protocol that is not followed leaves the patients susceptible to corneal exposure.
From review of the literature, suggestions that have been made for protecting the ocular surface include taping the eyes closed with transparent tape, eyelid closure, moisture chamber, covering the eyes with gauze, lubricants, normal saline irrigation of the eyes, and eye swabs to detect colonization if there is an infection elsewhere. A moisture chamber refers to using a substance such as polyethylene covers or swimming goggles to completely seal-off the eye from the environment.[28,33,34] In extreme cases, closure by tarsorrhaphy has been suggested, but it makes examination of the eyes difficult.[29]
Several studies have directly compared eye care practices. Lenart et al. studied 50 patients who each had one eye that received artificial tear ointment every 4 hrs while the fellow eye was passively closed by nurses when it was noted to be open. Among the 50 patients, there were nine abrasions in the passively closed eyes, compared with two abrasions in the ointment eyes (p = 0.004).[35] Similarly, Ezra et al. compared eye toilet with Geliperm (hydrogel dressing, Geistlich Pharma, Wolhusen, Switzerland) and Lacrilube (artificial tear ointment, Allergan, Inc., Irvine, CA). Although not defined in their article, eye toilet generally refers to regular cleaning of the eyes with sterile water.[36] Of the 24 patients in the eye toilet group, 13 (53%) had some degree of exposure keratopathy; of the 10 patients in the Geliperm group, nine (90%) had exposure keratopathy; and of the 13 patients in the Lacrilube group, two (15%) had exposure keratopathy. They concluded that Lacrilube is more effective at preventing keratopathy in this population than eye toilet (p = 0.04) or Geliperm (p = 0.001).[36] This comparison is of interest, because in 2003 a large survey showed that 75% of British ICUs use Geliperm, a product not designed for this purpose.[37]
Moisture chambers have been proposed as a way to protect the cornea even if the eye is open. Cortese et al. studied 60 critically ill patients with a limited or absent blink reflex. In half of the patients, lubricating drops were given every 2 hrs; in the other half, the eyes were covered with polyethylene film to create a moisture chamber. Eight of the 30 patients who received drops had staining of the cornea with fluorescein, indicating exposure keratopathy, compared with one of the 30 patients in the moisture chamber group (p < 0.05).[33] In another study, Koroloff et al. compared 110 ICU patients with reduced or absent blink reflex, dividing them into a group that received hypromellose drops and Lacrilube every 2 hrs and a second group that had polyethylene covers placed over the eyes to create a moisture chamber. The eyes of the patients in both groups were also cleaned every 2 hrs with saline. They found that zero patients had corneal ulceration in the polyethylene group, and four patients had ulceration in the hypromellose group. In this trial, the difference was not statistically significant (p = 0.12). The ease of application and the lower cost of polyethylene covers led them to make moisture chambers their standard preventive treatment for all unconscious patients in their ICU.[34]
Although Koroloff et al. did not find a significant difference in their patients, Sivasankar et al. did find a difference. One hundred forty-six patients were divided into two groups, one treated with a moisture chamber created with swimming goggles and gauze soaked in sterile water (closed chamber), and the other treated with ocular lubricants and a securing tape over the eyes (open chamber). Exposure keratopathy was present in 39 (32%) eyes of the open chamber group and 10 (8%) eyes of the closed chamber group (p = 0.001).[29]
Since moisture chambers and lubrication are two of the best-studied methods for preventing exposure keratopathy, we combined all of the relevant trials in a meta-analysis.[29,33,34] We decided to compare moisture chambers with ocular lubricants because ocular lubricants form the backbone of several evidence-based ICU eye care guidelines.[17,30,31] A quick overview of the literature indicated that moisture chambers could be a promising alternative.
Materials and Methods
Randomized controlled trials comparing methods of eye care for patients in ICUs were identified by electronic searches of the MEDLINE database from 1966 to 2006, EMBASE, and Google Scholar. Search terms included ophthalmology, eye diseases, cornea, eye, intensive care units, critical care, and randomized control trial. Bibliographies of retrieved articles were searched for further studies. Standard textbooks in ophthalmology, critical care medicine, and critical care nursing were also searched for additional trials. The search strategy revealed six trials. After scanning the abstracts, we found that three were randomized trials of moisture chambers vs. lubrication. These three trials were assessed for quality and were found to have well-defined interventions, control groups, and assessments of outcomes. These three trials (reviewed individually in the preceding section) were combined for evaluation by meta-analysis.[29,33,34] There were no observational trials of moisture chambers. The three trials eliminated compared artificial tears with passive closure,[35] a complex eye care regimen to eye toilet alone,[36] and a complex eye care regimen with Geliperm.[38]
The random effects model was used to combine results from individual trials and calculate estimates of the benefits associated with therapy. Repeating the analysis with a fixed effects model gave similar results. The meta-analysis was conducted according to the QUORUM guidelines.[39] We used the comprehensive meta-analysis program (Biostat, Englewood, NJ). We used the Cochrane Q statistic to assess heterogeneity. A summary odds ratio was calculated using a random effects model from the odds ratios and the 95% confidence intervals for the end point using Mantel-Haenszel methods.
Results
The three randomized trials enrolled a total of 294 patients.[29,33,34] Meta-analysis of these studies showed that rates of exposure keratopathy are significantly lower when moisture chambers are used to protect the eye compared with lubricating ointments. Eight of 113 (7.1%) patients in the moisture chamber group vs. 32 of 151 (21.2%) patients in the lubrication group developed exposure keratopathy, with a summary odds ratio of 0.208 (95% confidence interval 0.090-0.479, p < 0.001). The heterogeneity among the results of the randomized controlled trials was not statistically significant (p = 0.666).
Limitations
Although the meta-analysis showed a benefit for moisture chambers compared with lubrication, only three trials have been performed with limited sample size. Also, the type of moisture chamber used and the type of control treatment varied between the trials. None of the trials rigorously assessed compliance with the protocols. More randomized controlled trials are needed to evaluate the best method of eye care in the ICU. Finally, results achieved in studies may be difficult to replicate in clinical practice. Clinicians and family members may be loath to cover or close patients’ eyes if they are partially sedated with an intermittent blink reflex. Possible solutions include alternating lubrication and coverage between the two eyes, using a transparent covering, or uncovering eyes when patient interaction is desired. Studies are needed to determine the feasibility and efficacy of these approaches.
Special Conditions
This review does not discuss patients intubated for a short period of time, such as patients presenting for elective surgery. Eye care for intensive care patients suffering from burns, ocular trauma, recent ocular surgery, or toxic epidermal necrolysis are outside the scope of this review. These populations are likely at even higher risk for epithelial damage and microbial keratitis. Ophthalmologic consultation for help in management is suggested.
Conclusions
Patients in the ICU are at increased risk of exposure keratopathy which may progress to visual loss. This common problem is underappreciated by ICU clinicians. Meta-analysis showed that moisture chambers are significantly better than lubrication at preventing exposure keratopathy in ICU patients. Possible reasons favoring moisture chambers over lubrication include simplicity of application and easier maintenance, because the chambers do not have to be reapplied every 2 hrs. Also moisture chambers ensure protection of the entire cornea. ICU staff should be educated about the value of moisture chambers in protecting the eyes of their patients. With application of simple protocols, exposure keratopathy can be prevented, thus improving patient care in the ICU.
References
|
Some of the results of this trial were previously presented in abstract form: Jamie Rosenberg, MD, Lewis Eisen, MD, Rachel Bloom, BA, Jeffrey Berger, MD, John Kim, MD. Prevention of exposure keratopathy in the intensive care unit: A systematic analysis of randomized controlled trials. Invest Ophthalmol Vis Sci. 2007; 48.
Lewis A. Eisen, MD, Email: leisen@gmail.com
Disclosure: The authors have not disclosed any potential conflicts of interest.
Tags: News, Rianimazione




One Response to “Eye Care in the Intensive Care Unit: Narrative Review and Meta-analysis”
Trackbacks/Pingbacks
Leave a Reply
You must be logged in to post a comment.