Charles Chazot, MD Guillaume Jean, MD
Nat Clin Pract Nephrol ():, 2009. © 2009 Nature Publishing Group
The duration and frequency of hemodialysis was determined empirically when this therapy first came into use, and treatment was commonly three 8 h sessions per week by the end of the 1960s. Subsequently, however, the growing number of patients who required this therapy had to be reconciled with the shortage of equipment; therefore, dialysis time was decreased to three 4 h sessions per week. At the same time, on the basis of data from the first randomized controlled trial of dialysis—the National Cooperative Dialysis Study—Kt/Vurea was devised as the optimum measure of dialysis adequacy. Nowadays, although Kt/Vurea targets are fulfilled in an increasing number of patients, observational studies show that individuals on hemodialysis continue to experience a high rate of complications, including hypertension, left ventricular hypertrophy, cardiac failure, hyperphosphatemia, malnutrition and death. Although no randomized controlled trial has yet been published, observational data indicate that increasing hemodialysis time and/or frequency improves a number of these complications, especially the death rate. This Review outlines the advantages of longer and/or more frequent dialysis sessions and highlights the barriers to adoption of such regimens, which largely relate to economics, patient willingness, and organization of dialysis units.
Introduction
Since the early 1960s, patients with end-stage renal disease (ESRD) have benefited from hemodialysis, which prolongs their survival. The usual hemodialysis prescription is currently thrice-weekly sessions of 34 h each (conventional hemodialysis [CHD]); this regimen was gradually defined after the introduction of hemodialysis. The first dialysis session for Clyde Shields, who was the first patient to be treated with this modality, lasted for 76 h![1] At the end of the 1960s, chronic hemodialysis usually involved three 812 h sessions per week.[25] As the demand for therapy became overwhelming, the treatment time was shortened to three 4 h sessions per week, since the clinical outcomes of this regimen were considered to be acceptable.[6] However, a registry analysis by Degoulet et al.[7] in 1982 revealed a high incidence of intradialytic adverse effects and a high rate of cardiovascular mortality in patients on hemodialysis, although no direct relationship between hemodialysis practice and patient outcome was identified. Patients on dialysis are now known to have a significantly increased rate of death, mainly of a cardiovascular cause, compared with the general population,[8] and nephrologists who care for these individuals are actively looking for ways to improve their patients’ outcomes. To this end, a number of alternative dialysis strategies, such as short daily hemodialysis (SDHD), long nocturnal daily hemodialysis (LNDHD), long conventional hemodialysis (LHD) and hemodiafiltration, are currently being investigated. This Review summarizes the benefits of and barriers to adoption of these regimens.
Historical Importance of Dialysis Time Versus Dose
In the first randomized clinical trial of dialysis— the National Cooperative Dialysis Study (NCDS)[9]—patients were divided into four groups on the basis of blood urea nitrogen level and dialysis time. The statistical relationship between treatment time and patient outcome in this study was considered to be nonsignificant because the P value for this correlation was 0.056. On the basis of data from the NCDS, the concept of ‘dialysis dose’ was introduced in the form of the Kt/Vurea formula (where K = urea clearance, t = dialysis time and V = total body water), which uses urea as a marker of uremia. The concept of Kt/Vurea was a major breakthrough and became so popular that, for more than two decades, hemodialysis treatment adequacy has remained predicated on the capacity to clear low-molecular- weight uremic toxins. Several guidelines recommend minimum target values of Kt/Vurea, with the goal of delivering an adequate dialysis dose to all patients.[10,11]
As a result of the NCDS, dialysis time has not been considered to be a key factor in the dialysis prescription; however, more than 25 years later, the interpretation of the NCDS is now once more being actively discussed, with Gotch[12] as the coinventor and leading proponent of the Kt/Vurea formula and Twardowski[13] as his main opponent. To summarize their vivid debate, Gotch believes that the actual (as opposed to intended) distribution of patients across the arms of the NCDS should have clearly advantaged dialysis time over urea clearance; however, longer dialysis time had no clear benefit.[12] By contrast, to Twardowski, the Kt/Vurea formula suggests that dialysis time can be decreased as long as urea clearance is proportionately increased, but he does not believe that patient outcomes remain the same.[13]
The randomized controlled HEMO trial did not take dialysis time into account because of the belief that this parameter is not a key determinant of outcomes independent of its contribution to Kt/Vurea. This trial compared the effects of standard versus high Kt/Vurea and high versus low membrane flux on patient outcomes. No clear difference in outcomes was found for either comparison;[14] hence, it seemed that a high dialysis dose is of no benefit to the patient. However, this conclusion deserves to be reconsidered, given the poor health of patients on hemodialysis.
Lessons From Dopps and Other Observational Studies
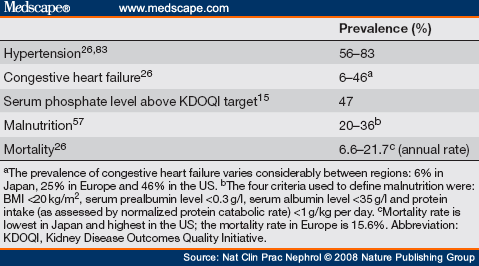
The Dialysis Outcomes and Practice Patterns Study (DOPPS) has provided a large amount of information on patients receiving hemodialysis all over the world, including data on complications and differences in practice. Recently, DOPPS investigators[15] showed that the mean dialysis prescription in the US in terms of Kt/Vurea has progressively increased during the past two decades, from 1.11 in 1991 to 1.52 in 2002. Between phase I (19962001) and II (2002 2004) of the DOPPS, the proportion of patients with a single-pool Kt/Vurea below 1.[2] decreased from 34% to 10%.[15] These data show that the great majority of patients fulfill the guideline recommendations for small-molecule clearance. At the same time, despite the large discrepancies between countries revealed by the DOPPS, rates of severe complications, such as hypertension, malnutrition, congestive heart failure, bone mineral disorders and death, remain high (Table 1). Thus, dialysis adequacy clearly has to take into account important factors beyond small-molecule clearance, such as extracellular volume (ECV) control, phosphate metabolism, nutrition and last but not least, patient survival, the indisputable end point. Alternative dialysis strategies involving longer and/or more-frequent treatment might provide a means of achieving this goal.
Cardiovascular Effects of Dialysis Time and Frequency
Ultrafiltration rate is defined as the interdialytic weight gain divided by the treatment time. In several studies, interdialytic weight gain was positively associated with nutritional markers and survival.[16,17] However, recent data stress the deleterious effect of a high ultrafiltration rate. DOPPS[18] data show that mortality is increased in patients with an ultrafiltration rate over 10 ml/kg per hour and that these individuals have a 30% greater risk of intradialytic hypotension. The relationship between ultrafiltration rate and mortality was confirmed in a 5-year prospective study by Movilli et al.,[19] who found that an ultrafiltration rate of over 12.4 ml/kg per hour was associated with worse survival, after multivariable adjustment.
The mechanism of the deleterious effect of high ultrafiltration rate is unknown, although hypotension episodes are related to high ultrafiltration rate (as described above) and Shoji et al.[20] reported that such episodes correlated with an increased risk of mortality in a cohort of 1,244 patients. In another study, hemodialysis-induced hypotension was associated with impaired aortic elasticity and decreased survival.[21] Alternatively (or additionally), patients with adverse effects of dialysis related to a high ultrafiltration rate (e.g. hypotension) might fail to achieve the prescribed dry weight and still have ECV overload (Figure 1)[22] as a result of decisions taken to treat the complication, such as stopping ultrafiltration, infusing saline, or increasing dry weight prescription or the dialysate sodium concentration. Inability to reach and maintain the dry weight exposes the patient to hypertension, left ventricular hypertrophy and congestive heart failure, conditions known to be associated with decreased patient survival.[23-25] As previously mentioned, congestive heart failure is an important comorbid condition in many patients on hemodialysis in Europe and the US (25-46% prevalence in the DOPPS report).[26] Toz et al.[27] showed a significant improvement in ejection fraction following fluid withdrawal in patients on hemodialysis who had severe heart failure; therefore, it is highly probable that inadequate handling of ECV excess contributes to the high prevalence of cardiac failure in the hemodialysis setting. Increasing dialysis time or frequency enables the ultrafiltration rate to be decreased. Increasing session time enables better session tolerance[18,28,29] and increasing frequency reduces the risk of dialysis-induced hypotension.[30]
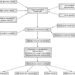 |
Figure 1. (click image to zoom) Relationship between ultrafiltration rate, dialysis-induced hypotension and extracellular volume overload, and the role of dialysis time and frequency.
|
Increasing dialysis time and frequency have other important cardiovascular effects in patients on hemodialysis, including correction of hypertension,[31-35] correction of left ventricular hypertrophy, [33-36] improvement of ejection fraction in individuals with heart failure,[27,36] reduction of peripheral resistance,[37] improvement of the vasodilatory response,[31] and reduction of sleep hypoxemia[38] (Box 1.). These beneficial effects are in part attributed to improvement of ECV control,[35,39] but the effects are also sometimes obtained without substantial changes in ECV.[40,41] This observation suggests that longer or more-frequent dialysis might increase the clearance of or reduce the exposure to toxins that affect the endothelium. However, Chan et al.[42] pointed out that restoration of endothelial reactivity by use of LNDHD was independent of asymmetric dimethylarginine plasma level. This molecule, which accumulates in patients with ESRD, alters the activity of nitric oxide synthase and thereby influences endothelium-mediated vasodilation. Whether LHD (as prescribed at our center in Tassin), SDHD or LNDHD is better for the cardiovascular system remains unknown at the moment, but this question might be answered in part by the ongoing Frequent Hemodialysis Network trials.[43]
Effects of Dialysis Time and Frequency on Phosphate Balance
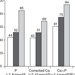
Block et al.[44] identified serum phosphate level as a prognostic factor in patients on hemodialysis. A high serum phosphate level increases the risk of vascular calcifications in vitro[45] and is associated with increased cardiovascular mortality in patients on hemodialysis.[46,47] Individuals on hemodialysis tend to have a high phosphate intake due to a high-protein diet, as nutritional recommendations call for such a diet to maintain nitrogen balance.[48] Gutzwiller et al.[49] thoroughly studied phosphate removal during hemodialysis. These investigators found that during the first 2 h, the level of phosphatemia decreases and phosphate removal is maximal; during the next 3 h, both the level of phosphatemia and phosphate removal remain stable. This observation clearly shows that the amount of phosphate removed during the dialysis session is time-dependent. Eloot et al.[50] analyzed patients treated with hemodialysis sessions of 4 h, 6 h, and 8 h, and reported that the amount of phosphate removed increased with session time and was significantly higher during the 8 h session than during the 4 h or 6 h sessions. Gotch and Levin[51] modeled phosphate balance according to protein intake, dialyzer clearance, and session time and frequency. The investigators showed that, in patients who ingested the recommended daily amount of protein, phosphate balance is likely to remain positive with CHD (sessions of 34 h) even when using a dialyzer with a high phosphate clearance, whereas phosphate removal by LHD (sessions of 68 h) should be sufficient to produce a neutral phosphate balance. These predictions are confirmed by data from Photo-Graph®, a French registry for bone mineral metabolism in patients on hemodialysis, which show adequate phosphate balance and a lower incidence of hyperparathyroidism in patients on LHD in Tassin (mean treatment time 6 h 20 min ± 1 h 15 min) than in those on CHD in the Rhône-Alpes area of France (mean treatment time 4 h ± 30 min), despite reduced administration of phosphate binders in the former (Figures 2 and 3).
Gotch and Levin also created another model, which showed that LNDHD provides moreeffective phosphate removal than does SDHD.[51] This prediction has been clinically confirmed by Lindsay et al.[52] who found nonsignificant decreases in serum phosphate level and phosphate binder use after the switch from CHD to SDHD, whereas the switch from CHD to LNDHD resulted in achievement of a normal serum phosphate level and enabled the discontinuation of phosphate binders. In another study by Gotch et al.,[53] patients treated with SDHD surprisingly required more phosphate binders than did those on CHD, although SDHD removed 21% more phosphate than CHD. These findings were confirmed by Kumar et al.[54] who noted better nutritional status, but increased use of phosphate binders, in 12 patients who switched from CHD to SDHD and were followed up for 3 years. These observations are explained by the substantial increases in protein and phosphate intakes that are seen in patients who receive SDHD, as discussed in the next section.
Dialysis time seems to be the most important factor for phosphate clearance. Thrice-weekly LHD—and particularly LNDHD—provides adequate control of bone mineral metabolism and reduces the need for phosphate binders. Increasing frequency alone by switching to SDHD might have a limited effect on phosphate control, especially in patients with a high protein intake. In a study by Ayus et al.,[55] SDHD significantly improved phosphatemia and increased phosphate removal compared with CHD, but the weekly treatment time was higher in the SDHD group (18 h vs 12 h).
Effects of Dialysis Time and Frequency On Nutrition
The HEMO study has provided a very clear picture of the nutritional status of patients on CHD.[56] During the 36-month follow-up period of this trial, patients in all four arms exhibited a progressive decline in body weight, serum albumin level and protein intake, as assessed by the normalized protein equivalent of nitrogen appearance (nPNA). Furthermore, in a crosssectional study by Aparicio et al.,[57] 20-36% of patients on CHD presented with at least one criterion for malnutrition. Alternative dialysis strategies seem to have more-positive nutritional effects than CHD. Patients who were switched from CHD to LHD had increased their body weight in a few months,[58,59] whereas patients whose treatment time was reduced from 8 h to 5 h had, after 1 year, a dry weight reduction of 2.5 kg and an increase in predialysis mean arterial pressure.[59] Moreover, in contrast to the HEMO study, our group has shown that food intake, nPNA, body weight and serum albumin level remained stable for 5 years in prevalent hemodialysis patients who were treated with LHD.[60] In a report by Galland et al.,[61] patients who switched from CHD to SDHD experienced dramatic increases in food intake, serum albumin and prealbumin levels, body weight and lean body mass after 1 year. As previously mentioned, Gotch et al.[53] reported increased protein intake in patients on SDHD compared with CHD. Improvements in amino acid profile have been described in patients who switched from CHD to LNDHD,[62] but in another study, Spanner et al.[63] reported no significant increase in nPNA and a decrease in serum albumin and prealbumin levels with LNDHD. Increasing dialysis time or frequency improves patients’ appetites and LHD provides nutritional stability. Why no consistent improvement in nutritional parameters has been reported when both time and frequency are increased is unclear.
Why do alternative dialysis strategies improve nutritional stability? The Modification of Diet in Renal Disease study showed that protein intake decreases as glomerular filtration rate declines,[64] and Bergström demonstrated in rats that uremic plasma contains anorectic compounds in the range of 15 kDa.[65] Accumulation of these anorectic compounds could contribute to malnutrition. LHD might remove these molecules more efficiently than CHD, and SDHD might reduce patient exposure to the molecules. This hypothesis is supported by a recent DOPPS analysis, in which appetite was found to be related to session time.[66] Eloot et al.[50] have shown that the removal of middle molecules, such as β2-microglobulin, increases with session time. By use of computer simulation, Goldfarb- Rumyantzev et al.[67] showed that SDHD increases the β2-microglobulin clearance only slightly compared with CHD, whereas clearance of this molecule is significantly increased with LHD compared with CHD.
Effects of Dialysis Time and Frequency on Survival
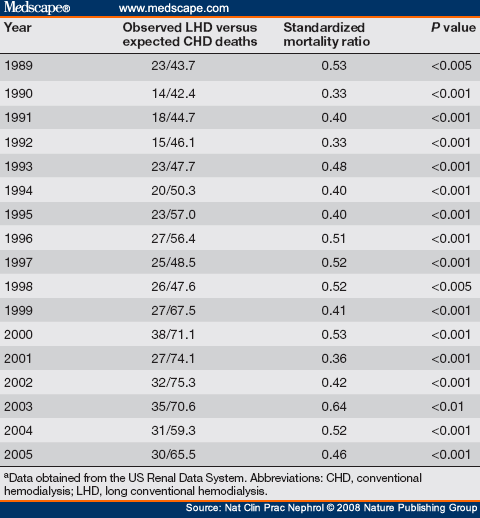
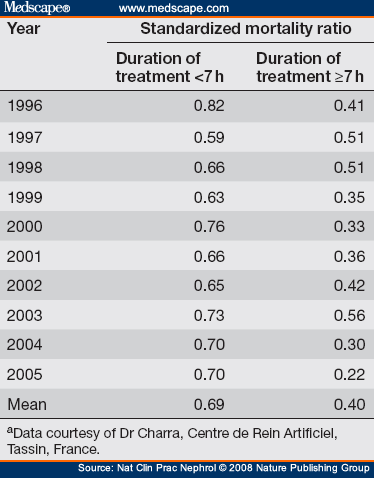
Observational data suggest that the positive effects of prolonged dialysis time or increased dialysis frequency translate into improved patient survival. In Tassin, every year for more than a decade, we have compared the mortality of our patients on LHD to that of US patients on CHD, by use of data from the US Renal Data System.[68] For each calendar year and despite the worsening of the case-mix over time, LHD has been associated with a survival advantage of approximately 50% (Table 2). This finding is dependent on treatment time (Table 3); patients treated for at least 7 h per session have better survival than those patients treated for shorter times. Observational data from the US, Japan, Australia and New Zealand confirm the importance of treatment time for survival.[69-71] Saran et al.[18] also reported a significant effect of session time on patient survival in the DOPPS. Compared with patients who had a thrice-weekly session time greater than 240 min, the adjusted risk of dying was 19% higher in patients treated for 211 240 min and 34% higher in patients whose sessions lasted less than 211 min. The time benefit varied among the continents—being greatest in Japan, intermediate in Europe and lowest in the US—but it was significant in all cases. Moreover, this study found a significant synergistic effect of time and Kt/Vurea. Although no mortality data have yet been reported for LNDHD,[72] experience from five centers in the US, Italy, France and the UK of 415 patients on in-center or home SDHD shows that the standardized mortality ratio is 0.34 compared with patients in the US Renal Data System (Figure 4), and the survival rate is identical to that of deceased-donor kidney transplant recipients.[73]
The Frequent Hemodialysis Network is currently conducting two trials to compare CHD with in-center SDHD and home LNDHD in terms of mortality, left ventricular hypertrophy and quality of life.[43] No trial has so far compared LHD with CHD or SDHD with LNDHD.
Effects of Dialysis Time and Frequency on Other Factors
By mimicking physiological kidney function more closely than CHD, increasing dialysis time and/or frequency might provide benefits other than those reported above. Erythropoietin consumption is usually reduced by SDHD and LNDHD.[74,75] However, positive results are not consistently found with regard to this parameter, quality of life or cognitive function, and such data have usually been obtained from small groups that may or may not include controls.[76,77] No data are available on the effects of different dialysis strategies on male fertility, but six successful pregnancies have been reported in five women who underwent LNDHD at one center.[78] In the study, the rate of live birth (86%) and the average newborn weight (2,417.5 ± 657.0 g) were both higher than those obtained from pooled data that included 131 pregnancies in patients treated with various dialysis regimens (livebirth rate 70.9% and average newborn weight 1,483 ± 116 g).[79]
Barriers to Increasing Dialysis Time and/or Frequency
The ‘cons’ of increasing dialysis time and/or frequency are largely barriers to adoption of these alternative modalities rather than genuine disadvantages. The main barrier is the fact that no randomized controlled trial has yet demonstrated the clinical advantage of these strategies over CHD. As stated by Gotch,[12] only Kt/Vurea is known to affect patient outcome; hence, this formula remains the main tool for determining dialysis adequacy. Without denying the importance of Kt/Vurea, however, there is evidence that the formula does not take into account important factors such as control of ECV or phosphate balance. If these parameters are inadequate despite sufficient dialysis dose in terms of smallmolecule clearance, dialysis time and frequency are the only tools that can be used to improve the situation.
The second barrier to greater adoption of alternative dialysis regimens is patient willingness and compliance. Convincing patients to increase their session time and/or frequency is not easy. Patients might refuse to undergo home or in-center long nocturnal dialysis because they are concerned about safety issues or having difficulty sleeping, or are reluctant to leave their family or spouse during the night. When given the choice, 44% of patients declined to switch from CHD to SDHD, despite being informed of the expected health benefits.[80] Even patients who do elect to begin SDHD sometimes skip sessions.[81] The third barrier to adoption of alternative strategies is that these regimens can disrupt the organization of dialysis units and/or impose considerable staffing and economic burdens on the units. The final barrier, but by no means the least important, is that the economic benefit of alternative strategies has not yet been clearly analyzed by either the scientific community or health-care authorities. The reduced morbidity and prescriptions of medications associated with increasing dialysis session time and/or frequency would be a source of savings.[82] Nephrologists must convince health-care authorities and the owners of dialysis units to implement these alternative strategies, in the same way that they have been successful in some countries at defeating the restrictions on access of the elderly to dialysis.
Conclusions
Hemodialysis throughout the world generally involves three weekly sessions of 34 h each. Logistical and economic considerations have profoundly influenced this approach. Although some patients do well on this regimen, observational data indicate that serious complications are frequent in many individuals. These individuals clearly require a different dialysis prescription. For the past two decades, the influence of the Kt/Vurea formula has meant that the modification of dialysis prescriptions has focused on increasing the clearance of small molecules; however, this strategy has a limited effect and does not improve ECV control or phosphate balance. Even though we are still awaiting the results of current randomized controlled trials of SDHD and LNDHD, nephrologists should consider increasing dialysis time and/or frequency to improve the condition of patients on hemodialysis.
Patient education is crucial to bring about understanding and acceptance of prolonged or more frequent dialysis sessions. Provision of health education, exercise training or entertainment programs (e.g. books or internet use) during dialysis could help to improve patient compliance with this approach, and thereby improve outcomes. The other task for nephrologists is to convince the staff and owners of dialysis units to undertake the reorganization that is necessary to be able to offer longer and/or more frequent dialysis sessions. Different patients have various dialysis needs; therefore, think tanks that involve nephrologists, owners of dialysis units, nurses, other caregivers and patients should be convened to determine how best to achieve this reorganization. Last, but not least, costbenefit analyses are urgently needed to guide hemodialysis prescription and to facilitate discussion with health-care authorities, with a view to creating a fair and rational reimbursement policy for alternative dialysis strategies.
In our opinion, future clinical research should endeavor to establish which alternative modality—LHD, SDHD or LNDHD—has the most positive clinical and economic impact. This question will be answered in part by the Frequent Hemodialysis Network trials. However, it is possible that in many patients, extending dialysis sessions by just 12 h would be sufficient to resolve most complications and improve outcomes, at a lower cost and lower burden to the patient than SDHD or LNDHD. This theory remains to be tested.
Key Points
- Observational data suggest that increasing hemodialysis session time and/or frequency reduces the risk of complications
- Increasing dialysis session time and/or frequency reduces ultrafiltration rate, which contributes to the improvement of cardiovascular complications by the use of alternative dialysis strategies
- Phosphate removal is time-dependent, and long dialysis sessions help to achieve phosphate balance and reduce the need for phosphate binders; however, short daily dialysis has limited effect on phosphate control, especially in patients with high protein intake
- Appetite and nutritional markers are positively influenced by increasing dialysis time or frequency
- Randomized controlled trials comparing the effects of conventional and short daily or long nocturnal daily hemodialysis on mortality, left ventricular mass and quality of life are underway
- Nephrologists have the task of convincing both their patients and the managers of their dialysis facility to accept and implement high-frequency and/or prolonged dialysis regimens
Acknowledgements
The authors thank Chloë Harman, Locum Editor of Nature Clinical Practice Nephrology, for her editorial help. The authors are also deeply appreciative to the scientific committee of Photo-Graph® for authorizing the use of its mineral metabolism data. Désirée Lie, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Reprint Address
Charles Chazot, Centre de Rein Artificiel, 42 Avenue du 8 Mai 1945, 69160 Tassin, France; E-mail: chchazot@wanadoo.fr
Tables
Table 1. Prevalence of Clinical Complications in Patients on Hemodialysis
Table 2. Standardized Mortality Ratios[68] for Patients who Received Long Conventional Hemodialysis at the Centre de Rein Artificiel, Tassin, France, Compared With US Patients who Received Conventional Hemodialysisa
Table 3. Standardized Mortality Ratios for Patients Who Received Long Conventional Hemodialysis at the Centre de Rein Artificiel, Tassin, France (Compared With Us Patients Who Received Conventional Hemodialysis), according to prescribed duration of thrice-weekly treatment sessionsa
Box 1. Cardiovascular Parameters and Complications Reported to be Positively Affected by Alternative Hemodialysis Modalities Involving Increased Session Time and/or Frequency
References
- Scribner BH et al. (1960) The treatment of chronic uremia by means of intermittent hemodialysis: a preliminary report. Trans Am Soc Artif Intern Organs 6: 114122
- Thomson GE et al. (1967) Hemodialysis for chronic renal failure: clinical observations. Arch Intern Med 120: 153167
- Curtis JR et al. (1969) Maintenance haemodialysis. Q J Med 38: 4989
- Traeger J et al. (1969) Arterial hypertension in chronic kidney failure treated by extra-renal dialysis [French]. Acquis Med Recent 261296
- Vertes V et al. (1969) Hypertension in end-stage renal disease. N Engl J Med 280: 978981
- Cambi V et al. (1975) Short dialysis schedules (SDS)—finally ready to become routine? Proc Eur Dial Transplant Assoc 11: 112120
- Degoulet P et al. (1982) Mortality risk factors in patients treated by chronic hemodialysis: report of the Diaphane collaborative study. Nephron 31: 103110
- Levey AS et al. (1998) Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis 32: 853906
- Lowrie EG et al. (1981) Effect of the hemodialysis prescription of patient morbidity: report from the National Cooperative Dialysis Study. N Engl J Med 305: 11761181
- [No authors listed] (1997) NKF-DOQI clinical practice guidelines for hemodialysis adequacy: National Kidney Foundation. Am J Kidney Dis 30 (Suppl 2): S15S66
- Tattersall J et al. (2007) EBPG guideline on dialysis strategies. Nephrol Dial Transplant 22 (Suppl 2): ii5ii21
- Gotch F (2007) The basic, quantifiable parameter of dialysis prescription is Kt/V urea; treatment time is determined by the ultrafiltration requirement; all three parameters are of equal importance. Blood Purif 25: 1826
- Twardowski ZJ (2007) Treatment time and ultrafiltration rate are more important in dialysis prescription than small molecule clearance. Blood Purif 25: 9098
- Eknoyan G et al. for the Hemodialysis (HEMO) Study Group (2002) Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 20102019
- Port FK et al. (2006) Improving outcomes for dialysis patients in the international Dialysis Outcomes and Practice Patterns Study. Clin J Am Soc Nephrol 1: 246255
- Kimmel PL et al. (2000) Interdialytic weight gain and survival in hemodialysis patients: effects of duration of ESRD and diabetes mellitus. Kidney Int 57: 11411151
- Lopez-Gomez JM et al. (2005) Interdialytic weight gain as a marker of blood pressure, nutrition, and survival in hemodialysis patients. Kidney Int 67 (Suppl S93): S63S68
- Saran R et al. (2006) Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int 69: 12221228
- Movilli E et al. (2007) Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis: a 5-year prospective observational multicentre study. Nephrol Dial Transplant 22: 35473552
- Shoji T et al. (2004) Hemodialysis-associated hypotension as an independent risk factor for twoyear mortality in hemodialysis patients. Kidney Int 66: 12121220
- Duman D et al. (2008) Dialysis-induced hypotension is associated with impaired aortic elasticity in patients undergoing chronic hemodialysis. Blood Press Monit 13: 7378
- Charra B (1994) Control of blood pressure in long slow hemodialysis. Blood Purif 12: 252258
- Charra B et al. (1992) Survival as an index of adequacy of dialysis. Kidney Int 41: 12861291
- Fagugli RM et al. (2003) Association between extracellular water, left ventricular mass and hypertension in haemodialysis patients. Nephrol Dial Transplant 18: 23322338
- Goodkin DA et al. (2004) Mortality among hemodialysis patients in Europe, Japan, and the United States: case-mix effects. Am J Kidney Dis 44 (Suppl 2): S16S21
- Goodkin DA et al. (2003) Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 14: 32703277
- Toz H et al. (2007) Improvement in “uremic” cardiomyopathy by persistent ultrafiltration. Hemodial Int 11: 4650
- Brunet P et al. (1996) Tolerance of haemodialysis: a randomized cross-over trial of 5-h versus 4-h treatment time. Nephrol Dial Transplant 11: 4651
- Laurent G and Charra B (1998) The results of an 8 h thrice weekly haemodialysis schedule. Nephrol Dial Transplant 13: 125131
- Okada K et al. (2005) Prolonged protective effect of short daily hemodialysis against dialysis-induced hypotension. Kidney Blood Press Res 28: 6876
- Chan CT et al. (2003) Short-term blood pressure, noradrenergic, and vascular effects of nocturnal home hemodialysis. Hypertension 42: 925931
- Chazot C et al. (1995) Interdialysis blood pressure control by long haemodialysis sessions. Nephrol Dial Transplant 10: 831837
- Culleton BF et al. (2007) Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 298: 12911299
- Fagugli RM et al. (2006) Effects of short daily hemodialysis and extended standard hemodialysis on blood pressure and cardiac hypertrophy: a comparative study. J Nephrol 19: 7783
- Fagugli RM et al. (2001) Short daily hemodialysis: blood pressure control and left ventricular mass reduction in hypertensive hemodialysis patients. Am J Kidney Dis 38: 371376
- Chan CT et al. (2002) Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int 61: 22352239
- Katzarski KS et al. (1999) Fluid state and blood pressure control in patients treated with long and short haemodialysis. Nephrol Dial Transplant 14: 369375
- Chan CT et al. (2004) Impact of nocturnal hemodialysis on the variability of heart rate and duration of hypoxemia during sleep. Kidney Int 65: 661665
- Chazot C et al. (1999) The Janus-faced aspect of ‘dry weight’. Nephrol Dial Transplant 14: 121124
- Chan C et al. (2002) Improvement in ejection fraction by nocturnal haemodialysis in end-stage renal failure patients with coexisting heart failure. Nephrol Dial Transplant 17: 15181521
- Luik AJ et al. (2001) The influence of increasing dialysis treatment time and reducing dry weight on blood pressure control in hemodialysis patients: a prospective study. Am J Nephrol 21: 471478
- Chan CT et al. (2005) Dissociation between the shortterm effects of nocturnal hemodialysis on endothelium dependent vasodilation and plasma ADMA. Arterioscler Thromb Vasc Biol 25: 26852686
- Suri RS et al. for the Frequent Hemodialysis Network Trial Group (2007) Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int 71: 349359
- Block GA et al. (1998) Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31: 607617
- Giachelli CM (2003) Vascular calcification: in vitro evidence for the role of inorganic phosphate. J Am Soc Nephrol 14 (Suppl 4): S300S304
- Ganesh SK et al. (2001) Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 21312138
- Kalantar-Zadeh K et al. (2006) Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771780
- Fouque D et al. (2007) EBPG guideline on nutrition. Nephrol Dial Transplant 22 (Suppl 2): Sii45Sii87
- Gutzwiller JP et al. (2002) Estimating phosphate removal in haemodialysis: an additional tool to quantify dialysis dose. Nephrol Dial Transplant 17: 10371044
- Eloot S et al. (2008) Impact of hemodialysis duration on the removal of uremic retention solutes. Kidney Int 73: 765770
- Gotch FA and Levin NW (2003) Daily dialysis: the long and the short of it. Blood Purif 21: 271281
- Lindsay RM et al. (2003) Calcium and phosphate balance with quotidian hemodialysis. Am J Kidney Dis 42: 2429
- Gotch FA et al. (2003) A kinetic model of inorganic phosphorus mass balance in hemodialysis therapy. Blood Purif 21: 5157
- Kumar VA et al. (2007) Daily home hemodialysis at a health maintenance organization: three-year experience. Hemodial Int 11: 225230
- Ayus JC et al. (2007) Phosphorus balance and mineral metabolism with 3 h daily hemodialysis. Kidney Int 71: 336342
- Rocco MV et al. (2004) The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: results of the HEMO Study. Kidney Int 65: 23212334
- Aparicio M et al. (1999) Nutritional status of haemodialysis patients: a French national cooperative study: French Study Group for Nutrition in Dialysis. Nephrol Dial Transplant 14: 16791686
- Alloatti S et al. (2002) Long nocturnal dialysis. Blood Purif 20: 525530
- Charra B et al. (1998) Hemodialysis trends in time, 1989 to 1998, independent of dose and outcome. Am J Kidney Dis 32 (Suppl 4): S63S70
- Chazot C et al. (2006) Stability of nutritional parameters during a 5-year follow-up in patients treated with sequential long-hour hemodialysis. Hemodial Int 10: 389393
- Galland R et al. (2001) Short daily hemodialysis rapidly improves nutritional status in hemodialysis patients. Kidney Int 60: 15551560
- Raj DS et al. (2000) Plasma amino acid profile on nocturnal hemodialysis. Blood Purif 18: 97102
- Spanner E et al. (2003) The impact of quotidian hemodialysis on nutrition. Am J Kidney Dis 42: 3035
- Kopple JD et al. (2000) Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int 57: 16881703
- Bergstrom J (1996) Anorexia in dialysis patients. Semin Nephrol 16: 222229
- Lopes AA et al. (2007) Lack of appetite in haemodialysis patients—associations with patient characteristics, indicators of nutritional status and outcomes in the international DOPPS. Nephrol Dial Transplant 22: 35383546
- Goldfarb-Rumyantzev AS et al. (2002) Computer simulation of small-solute and middle-molecule removal during short daily and long thrice-weekly hemodialysis. Am J Kidney Dis 40: 12111218
- Charra B et al. (2004) Long thrice weekly hemodialysis: the Tassin experience. Int J Artif Organs 27: 265283
- Held PJ et al. (1991) Mortality and duration of hemodialysis treatment. JAMA 265: 871875
- Marshall MR et al. (2006) Associations of hemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney Int 69: 12291236
- Shinzato T et al. (1997) Survival in long-term haemodialysis patients: results from the annual survey of the Japanese Society for Dialysis Therapy. Nephrol Dial Transplant 12: 884888
- Walsh M et al. (2005) A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int 67: 15001508
- Kjellstrand CM et al. (2008) Short daily haemodialysis: survival in 415 patients treated for 1006 patient-years. Nephrol Dial Transplant 23: 32833289
- Pierratos A (2004) Daily nocturnal home hemodialysis. Kidney Int 65: 19751986
- Punal J et al. (2008) Clinical effectiveness and quality of life of conventional haemodialysis versus short daily haemodialysis: a systematic review. Nephrol Dial Transplant 23: 26342646
- Kurella M et al. (2005) Frequent hemodialysis and psychosocial function. Semin Dial 18: 132136
- Kutner NG (2004) Quality of life and daily hemodialysis. Semin Dial 17: 9298
- Barua M et al. (2008) Successful pregnancies on nocturnal home hemodialysis. Clin J Am Soc Nephrol 3: 392396
- Chou CY et al. (2008) Pregnancy in patients on chronic dialysis: a single center experience and combined analysis of reported results. Eur J Obstet Gynecol Reprod Biol 136: 165170
- Halpern SD et al. (2004) Willingness of patients to switch from conventional to daily hemodialysis: looking before we leap. Am J Med 116: 606612
- Goldfarb-Rumyantzev AS et al. (2006) A crossover study of short daily haemodialysis. Nephrol Dial Transplant 21: 166175
- Mohr PE (2001) The economics of daily dialysis. Adv Ren Replace Ther 8: 273279
- Davenport A et al. (2008) Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int 73: 759764
- Gunal AI et al. (2002) Paradoxical rise in blood pressure during ultrafiltration is caused by increased cardiac output. J Nephrol 15: 4247
- Ayus JC et al. (2005) Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: a prospective, controlled study. J Am Soc Nephrol 16: 27782788
- Nesrallah G et al. (2003) Volume control and blood pressure management in patients undergoing quotidian hemodialysis. Am J Kidney Dis 42: 1317
- Zilch O et al. (2007) Sympathetic hyperactivity in haemodialysis patients is reduced by short daily haemodialysis. J Hypertens 25: 12851289
- Pierratos A et al. (2006) Daily hemodialysis 2006: state of the art [Italian]. Minerva Urol Nefro 58: 99115
Authors and Disclosures
As an organization accredited by the ACCME, Medscape, LLC requires everyone who is in a position to control the content of an education activity to disclose all relevant financial relationships with any commercial interest. The ACCME defines “relevant financial relationships” as financial relationships in any amount, occurring within the past 12 months, including financial relationships of a spouse or life partner, that could create a conflict of interest.
Medscape, LLC encourages Authors to identify investigational products or off-label uses of products regulated by the US Food and Drug Administration, at first mention and where appropriate in the content.
Author
Charles Chazot, MD
Nephrologist, Centre de Rein Artificiel, Tassin, France Disclosure: Charles Chazot, MD, has disclosed that he has served as a consultant for Fresenius Medical Care.
Guillaume Jean, MD
Nephrologist, Centre de Rein Artificiel, Tassin, France Disclosure: Guillaume Jean, MD, has disclosed that he has served as a consultant for Fresenius Medical Care.
CME Author
Désirée Lie, MD, MSEd
Clinical Professor, Family Medicine, University of California, Orange; Director, Division of Faculty Development, UCI Medical Center, Orange, California Disclosure: Désirée Lie, MD, MSEd, has disclosed no relevant financial relationships.
Editor
Chloe Harman
Locum Editor, Nature Clinical Practice Nephrology
Disclosure: Chloe Harman has disclosed no relevant financial relationships.
Tags: News, Rianimazione

![Cumulative survival of patients who received hemodialysis (data obtained from the US Renal Data System [2005]) and patients treated with short daily in-center or home hemodialysis (data pooled from five centers in the US, Italy, France and the UK).](http://images.medscape.com/pi/editorial/journalcme/2008/17769/thumb-chazot.fig4.gif)





2 Responses to “The Advantages and Challenges of Increasing the Duration and Frequency of Maintenance Dialysis Sessions”
Trackbacks/Pingbacks
Leave a Reply
You must be logged in to post a comment.