Teresa J. Nel Transfusion Alter Transfusion Med. 2008;10(2):61-69. ©2008 Blackwell Publishing
Posted 10/07/2008
Abstract
The transfusion of blood and blood components is a critical element for the delivery of a healthcare service to patients. Tools to help improve the safety of the blood supply of a country include: (i) clinical (transfusion) guidelines to direct transfusion practices; (ii) audit systems to monitor adherence to the guidelines as well as the effects of adjustments to the guidelines; and (iii) the hemovigilance program which monitors the entire blood supply value chain, develops measures and solutions to problems that threaten or might threaten the safety of blood component recipients, and monitors the implementation of these corrective actions. Establishing guidelines, implementing audit systems and hemovigilance programs are achieved more readily in developing countries with limited resources available, if a step-up approach is used. If these tools are instigated on an inappropriate level as initial programs, they will be unattainable and most initiatives would fail because the performance targets are unrealistically high. Starting with an elementary approach, acknowledging the shortcomings of it while creating a culture of continuous improvement, is the preferred option.
Introduction
The transfusion of blood components continues to save millions of lives across the world. If used appropriately, blood transfusion is an extremely cost-effective life-saving measure. In developed countries such as the United States of America, it has been demonstrated that the approximate 2% of the healthcare budget spent on Blood Transfusion Services benefits 50% of the total healthcare services.[1]
Blood components are living human tissue and their administration is not without risk. The safety of the blood component that is delivered to the patient is thus of critical concern. To ensure the safety of the blood supply, there must be systems in place that will evaluate, monitor and manage risk along the entire blood supply value chain (Figure 1). Critical components to ensure this include: establishing guidelines to ensure the appropriate use of blood components, setting up auditing systems to monitor the usage of blood components and developing hemovigilance programs to provide an independent oversight along the entire blood supply chain.
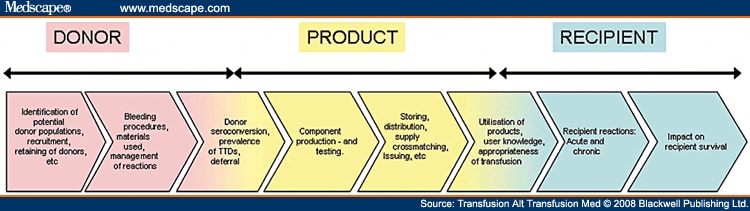
Figure 1.The blood supply value chain.
Each of these systems has a different focus: the aim of clinical transfusion guidelines is to give direction to the clinician on when to transfuse and what the expected outcome should be. Guidelines are therefore a tool to ensure appropriate utilization of blood products. This is of critical importance in light of possible transfusion complications and a dwindling donor base. The audit process can measure the appropriateness of the transfusion that did take place or is anticipated, against the set guidelines. The audit process and guidelines pertain to the clinical arm of the blood supply chain (Figure 2). Hemovigilance monitors all adverse events across the entire blood supply chain and provides a comprehensive oversight of the blood supply chain.
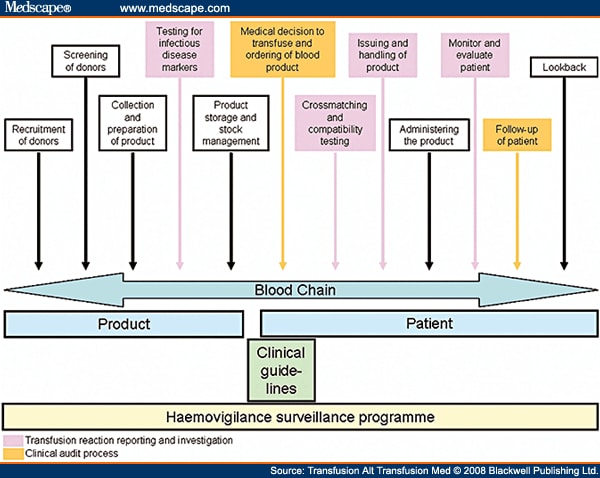
Figure 2.The scope of guidelines, audits and hemovigilance.
Clinical Guidelines
Clinical transfusion guidelines are tools used to aid clinicians with the appropriate use of blood components in the treatment of patients. These guidelines are not absolute rules but will rather guide the clinician to utilize the most appropriate blood component in the treatment of the patient if and when a transfusion is indicated. Each patient must be evaluated individually and, if justified, the clinician can adjust the treatment guidelines for a specific patient. The decision to transfuse is therefore based ultimately on a clinical assessment of a specific patient’s condition and appropriate laboratory parameters. Transfusion therapy must always be of overall benefit to the patient.
Developing Clinical Transfusion Guidelines
The ultimate aim of any country should be to have national guidelines on the clinical use of blood components. The problem is the time and resources needed to develop such guidelines. Hospitals in a developing country might consider utilizing transfusion guidelines available in the transfusion literature as background information to adapt and thereby establish local guidelines. The local circumstances in terms of patient populations served, level and standard of healthcare services available to patients, availability of safe blood components and type of components available will play an important role in establishing appropriate guidelines for local circumstances. If guidelines from developed countries are therefore used as a basis for the establishment of guidelines in a developing country, these must be adapted to take cognizance of the factors listed above. The World Health Organization has developed documentation to assist developing countries with establishing transfusion guidelines.[2]
Input for establishing guidelines must be obtained from the clinicians as well as the blood transfusion service. The critical element when drafting these guidelines must be that blood components will only be transfused for those conditions that are associated with significant morbidity and mortality and for which there is no other preventive or effective treatment. The guidelines must be practical and relevant to local conditions to ensure appropriate and cost-effective treatment for those circumstances when blood components are needed.
Basic Clinical Transfusion Guidelines
The following outlines the basic functions of the various blood products and should be the basis of the standards that will be set. Regarding the decision to transfuse:
- Red cell use should be based primarily on the patient’s risk for complications owing to inadequate oxygenation of tissue.
- Platelet use should be based on the risk for bleeding complications owing to qualitative and/or quantitative platelet disorders.
- Fresh frozen plasma use should be based on the risk for bleeding complications owing to qualitative and/or quantitative clotting factor disorders.
An example of transfusion guidelines for the red cell products in adult patients is given in Table 1 .[3]
Clinical guidelines do have shortcomings which must be considered. First, guidelines cannot define absolute transfusion triggers. For red cell transfusions, hemoglobin or hematocrit levels are mostly used to indicate when a patient should be transfused. These measurements do not reflect the degree of tissue oxygenation at a given point in time. Transfusion guidelines may also promote unnecessary transfusions if they are too liberal. On the other hand, guidelines should not be too restrictive, as this may result in an unacceptable number of transfusions where the criteria set by the guidelines are not met.
Once guidelines have been developed, these must be tested within the clinical setting of patient care and there must be a review process which allows for adjustments or changes to the guidelines. Auditing of transfusion practices is also a helpful tool for evaluating the appropriateness of the transfusion guidelines that are in use.
Audit of Transfusion Practices
The transfusion audit (i.e. clinical audit) is a quality improvement process that seeks to improve patient care and outcomes, through the systematic review of the use of transfused blood components against transfusion guidelines. The audit is a systematic, critical analysis of the quality of care which will include the procedures used for the diagnosis and treatment of the patient, the use of available resources, as well as the outcome and change in the quality of life of the recipients.[4] The aim of this process is to create a culture of delivering a quality service to patients whereby medical care will be improved on a continuous basis.
Auditing transfusion practices is therefore a cyclical process (Figure 3). Within the cycle, there are stages that follow a systematic process of establishing best practice taking into account local circumstances and limitations, measuring care against established criteria, taking action to improve care given and monitoring to sustain improvement. The spiral suggests that as the process continues, each cycle aspires to a higher level of quality.[5]
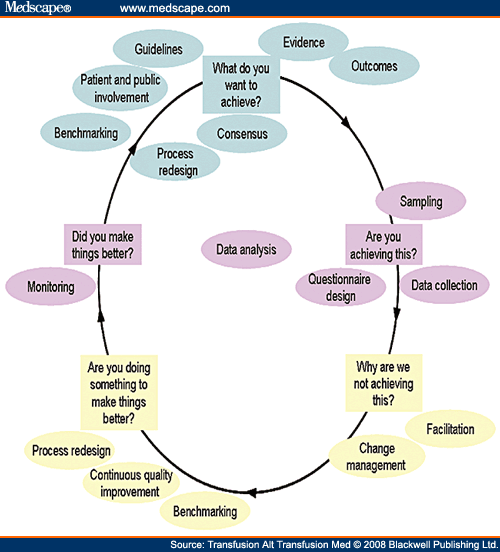
Figure 3.The audit cycle.
Transfusion audits can become a complex and time-consuming activity. Depending on the criteria used, a rather extensive amount of information may be needed about each transfusion to determine whether it met the criteria specified for the blood component that was transfused. Furthermore, the number of transfusion episodes that are reviewed to determine whether the use of the blood component was consistent with the guidelines will also influence the extent of the audit. In countries with limited resources, an audit must be planned carefully in order to keep it manageable and to gain the most benefit from it.
Owing to the cyclic nature of an audit program, a very elementary approach can be used for the first audit cycle. As confidence is gained, the audit process can be extended to a higher level. Once results are obtained from the initial audits, they can also serve as tools to motivate for increased resources to be allocated to the process.
The Audit Process
The clinical audit’s aim is to assess the following in terms of blood usage and the outcome of the transfusions given:[4]
- The hospital’s or department’s transfusion rate (i.e. the number of units transfused per hospital bed per year).
- The percentage transfused per user or user department for each indication.
- Crossmatch/transfusion ratio per user or user department for type of surgery and/or indication.
- The transfusion failure rate, i.e. the percentage of transfusions that did not achieve the expected outcome or result.
- The incidence of non-compliance with transfusion guidelines.
Transfusion triggers are measures that can be used for initial audits. The benefit of these measures is that they do not need a very sophisticated healthcare service to supply the tests that are used. Transfusion triggers that might be useful are the following:
- For red cells:
- Hemoglobin or hematocrit levels.
- Clinical parameters such as pulse rate and blood pressure (baseline and post-transfusion).
- For platelets:
- Platelet count.
- The presence or absence of clinical bleeding.
- For fresh frozen plasma:
- Results of coagulation tests (prothrombin time or international normalized ratio and activated partial thromboplastin time).
- For all blood components:
- The reason/s for the transfusion and operation (if applicable).
These measures do have shortcomings that should be recognized, in that limited clinical information is included, but the measures will give an indication of how well the clinicians adhere to the guidelines in well-controlled circumstances. It will also serve as a simple way to start off with auditing in settings with limited resources and start to create a culture of improving the level of care on a continuous basis. A further advantage is that it will not put a huge demand on existing resources within a resource-poor setting.
Audit Strategies
Transfusion audit data may be gathered retrospectively, prospectively or concurrently.[6] One of the methods used often in areas with limited resources is retrospective audits. In this type of audit, information regarding transfusions given to patients is gathered and reviewed some time after the transfusion episode and subsequent discharge of the patient. The review is therefore retrospective by necessity and the information is used in an attempt to alter clinicians’ behavior in the future.[6] Clearly, it does not have any impact on the treatment of the patients that were included in the audit.
Retrospective audits do have limitations, mostly owing to the fact that appropriate recording of all the necessary information is very often lacking. The advantage of this type of audit is that it does not need many resources to be implemented and maintained. It can also be managed on a manual system. This type of audit strategy is capable of yielding some information on what current practices consist of and will give guidance regarding strategies for clinicians’ education. It also serves as a tool to establish whether current transfusion practices are acceptable in terms of the agreed guidelines and whether the guidelines are at an acceptable standard.
A second type of strategy is that of prospective auditing. This entails reviewing and validating the decision to transfuse at the time when it is made against the agreed clinical guidelines. It therefore implies the review of orders for blood prior to the transfusion episode. This approach needs considerably more resources than a retrospective audit approach. Somebody with transfusion knowledge and experience must be available constantly to monitor transfusion practices and must also have access to laboratory results on a continuous basis. The advantage of this approach is that it gives the patient the benefit of receiving the appropriate transfusion therapy. It further allows for much more accurate information to be gathered for extensive audit evaluations.
A third option is concurrent audits which entail gathering information on a transfusion episode and giving feedback within the time scale of a patient’s stay in the hospital. The advantage of this approach is that the data are likely to be accurate and the outcome can be provided to the clinicians making the decisions regarding the transfusion episode while the specific incident can still be recalled. The disadvantage is, as is the case with retrospective audits, that this type of audit will not influence the transfusion decision for the current patient.
Comparative Audits
When starting an audit system, it might be easier to begin with one hospital or hospital department. It is however beneficial to add more hospitals as this will allow for comparisons in terms of transfusion rates. It might also be possible to compare audit results with published results from the literature. The problem with this is that most of these types of publications originate from developed countries. This impact on differences in the patient population, type of surgery done, diseases treated, facilities available, expertise available, etc., and will influence the outcome of the audits. This is an important factor that must be taken into account.
Feedback
Feedback on the audit should take the format of an audit report and this should be presented to a body that has the authority to implement recommendations.
Hemovigilance
Well-established hemovigilance programs have been in place for a number of years in some developed countries.[7] The Serious Hazard of Transfusion program of the United Kingdom, for instance, includes data on transfusion reactions since 1996. At the time when these programs originated, hemovigilance was seen as a composite of measures collected and evaluated in a systematic way on expected and unexpected or unwanted effects and outcomes in recipients, as a result of the clinical usage of blood and blood products.
Over the last decade, hemovigilance programs have evolved in developed countries to fulfilling an active vigilance function, which monitors all elements in the blood supply value chain (Figure 1), develops measures and solutions to problems (anywhere along the chain) that threaten or might threaten the safety of the recipients of blood, and monitors the implementation of these corrective actions.
Definition
Hemovigilance comes from ‘haema’, the Greek word for blood and ‘vigilance’ from Latin, which means ‘paying particular attention to’.[8] There are different definitions used to describe hemovigilance. The European Union Directive, for instance, defines it as: ‘A group of organized surveillance procedures relating to serious adverse or unexpected events or reactions in donors or recipients and the epidemiological follow-up of donors’.[9] The European Hemovigilance Network’s definition is the one most widely used and it states: ‘Hemovigilance is a set of surveillance procedures covering the entire transfusion chain (from the donation of blood and its components to the follow-up of recipients of transfusions), intended to collect and assess information on unexpected or undesirable effects resulting from the therapeutic use of labile blood products, and to prevent the occurrence or recurrence of such incidents’.[8]
The objectives of a hemovigilance program are among others to provide documented evidence for improvement of practice, to know what the real risks/hazards of transfusion are in a given community/country, to disseminate the findings and to take appropriate action as well as instigate appropriate education processes to prevent their occurrence and reoccurrence.[8]
Hemovigilance is therefore responsible for examining the safety of the blood supply from outside the routine operations of the organizations that either supply or use blood products to treat patients. This function of a vigilance system in terms of blood components has been well recognized by many developed countries. This is evident by the incorporation of these programs into legislation pertaining to the delivery of blood transfusion services. Hemovigilance programs are a key element in, for instance, the European Union legislation pertaining to blood transfusion activities. In this legislation, as is the case for the Canadian, Quebec, and Australian systems, problems in terms of the blood supply value chain (Figure 2) are identified from the donor all the way to the recipient, root-cause analysis is carried out, and recommendations to prevent or reduce these problems/reactions are made to the various authorities responsible for blood transfusion, in these countries.
Scope of Hemovigilance Programs
The scope of hemovigilance has evolved since the time when it was first introduced. Initially, the main purpose of hemovigilance programs internationally was to collect on a national basis data on the number of transfusion reactions. A transfusion reaction is any unfavorable event occurring in a patient during or after blood transfusion.[10] About 0.5–3% of all transfusions result in some adverse events, but the majority of them are minor reactions with no significant clinical consequences.[11] In general, transfusion-related adverse events are categorized as infectious and non-infectious. However, there are other classifications in the literature based on time of occurrence (i.e. acute vs. delayed) or physiological mechanism (i.e. immune mediated vs. non-immune mediated).[11,12] Current trends in the evolution of hemovigilance programs include root cause analysis of identified problems, development of solutions to these problems, compilation of recommendations to statutory bodies whose function it is to oversee blood transfusion and monitoring the effectiveness of the implementation of corrective actions.
Hemovigilance is therefore a ‘quality process’ with the aim to improve quality and increase the safety of blood transfusion, taking into account that hemovigilance covers and surveys all activities of the blood transfusion chain from donors to recipients (Figure 2).[8] Hemovigilance identifies factors throughout the process that may be related to risk. Hemovigilance plays a critical role in ensuring that laboratory and clinical blood transfusion practice is optimal.
Hemovigilance contributes to improving the safety of the blood supply by:
- Providing the medical community with a reliable source of information about untoward effects of blood transfusion.
- Indicating corrective measures required to prevent the recurrence of some accidents or dysfunctions in the transfusion process.
- Warning healthcare workers and hospitals, as well as the blood transfusion services, about adverse events that could involve more individuals than just the index case.
- Informing blood users and stakeholders about policy.
- Improving standards.
Quality Systems Versus Hemovigilance
The quality system of an organization ensures that internal processes adhere to current requirements, including policies and procedures and internal standard operating procedures or working documents. It further ensures that current good laboratory practice and current good manufacturing practice are followed through its quality control procedures. This ensures that within a blood transfusion service there is document control and traceability, internal control over processes and evaluation of these in terms of the set policies and standards. The quality program does not evaluate the risks to the blood supply at a strategic level and does not apply vigilance to ensure that there is continuous monitoring of all aspects that play a role in the blood supply value chain. The quality systems of an organization such as the blood transfusion service are an internal organizational function and do not address the delivery of a service from outside the organization. Furthermore, as it is limited to the blood transfusion service’s processes, it is not required to take into account other role players in the blood supply value chain such as healthcare providers, who have their own quality systems in place to look at their own internal processes.
Developing a Hemovigilance Systems With Limited Resources
The best approach to start a hemovigilance program is again to opt for a stepwise implementation of the program. The approach that was followed in South Africa was to start of with identifying the hazards of transfusion which potentially has the greatest risk to a recipient in this country. A manual process was instigated whereby information was accumulated on a retrospective basis. Questionnaires were sent to the various blood transfusion services in the country collecting information on the following serious reactions:
- Incorrect blood products transfused (misdirected transfusions).
- Acute hemolytic reactions other than that caused by incorrect blood products transfused (excluding fever and mild allergic reactions).
- Delayed hemolytic reactions.
- Transfusion transmissible diseases.
- Transfusion-associated graft-versus-host disease.
- Transfusion-related acute lung injury (TRALI).
- Post-transfusion purpura.
The data that were collected in the first 4 years of the program were cumulative numbers and did not include much clinical information. Reports were compiled and these were presented to the Minister of Health, provincial departments of health, healthcare centers and clinicians. The education process that was developed subsequently to the first two reports focused mainly on incorrect blood products transfused, the safety of the supply in a country with a high human immune virus prevalence in the general population and how to identify TRALI.
The second phase of the hemovigilance program (2004) was to start collecting recipient information at the time of the reaction. At that stage, the services of a half day nursing sister was acquired to help with the collection of data. A manual system consisting of forms which included questions on relevant clinical data was distributed to all the hospitals and blood transfusion services. The nursing sister also set up a program to visit the hospitals and transfusion services from which the most reactions were reported to educate them on the process of supplying information to the hemovigilance program.
Results
Hemovigilance programs across the world, demonstrate that the most common serious error related to blood transfusion is the wrong blood component given to a patient.[7,11] The transfusion of an incorrect blood component is either when a patient receives a blood component that did not meet the appropriate specifications or when the component was intended for another patient. These incidents can be ascribed to human error and are therefore preventable. The South African data do not reflect this ( Table 2 ).[13,14] One of the reasons might be the way the data are collected; another reason might be that staff reports this as an acute hemolytic reaction owing to fear of prosecution.
On incorrect blood products transfused, the first reports from South Africa demonstrated that most of the errors were taking place at the bedside. In the 2004 report, there was an indication that this is shifting to the laboratory side. This was confirmed in the 2006 report. The next step now must be to develop a program that specifically looks at where in the laboratory these mistakes most often happen and why. A strategy will then have to be developed on how to address the problems and prevent the mistakes.
Furthermore, after the education processes which were started in 2001 and 2002, doctors did start to recognize TRALI-associated reactions ( Table 2 ). No post-transfusion purpura or transfusion-associated graft-versus-host disease was reported so far. These are relatively rare and the focus is still on the education process around the incorrect blood products being transfused and emphasizing the safety of the blood supply in terms of transfusion transmissible agents.
Conclusion
Guidelines, audits and hemovigilance have become an integral part to ensure the safety of the blood components that are used in the treatment of patients. These systems tend to be very sophisticated, but can also be implemented successfully in developing countries on a limited basis. This will improve the level of transfusion medicine practiced and the care given to patients. Developing guidelines, audit systems and hemovigilance programs in countries with limited resources can be achieved more readily if a step-up approach is used. If these tools are instigated on an inappropriately high level as initial programs, they will be unattainable and most initiatives will fail. The reason for this is that the performance targets would simply be beyond the reach of a resource-limited environment. It is better to start with an elementary approach, acknowledge the shortcomings of it, while creating a culture of continuous improvement, ultimately to the benefit of patients dependent on a safe and efficient blood supply.
CLICK HERE for subscription information about this journal.
Table 1. Guidelines for Adult Red Cell Transfusion
- Symptomatic anemia
- Acute blood loss > 500 mL
- Clinical shock with concurrent or recent bleeding (within 24 hours)
- Anemia with hemoglobin level < 8 g/dL (hematocrit level < 25%) that is not caused by iron, folate or vitamin B12 deficiency
- Anemia with hemoglobin level < 10 g/dL (hematocrit level < 30%) and one or more of the following:
- Coronary artery disease
- Cerebrovascular disease
- Chronic obstructive pulmonary disease or hypoxemia (PO2 < 60 mmHg on room air)
- Tachycardia (pulse > 100 beats/minute) with temperature < 100°F (37.8°C)
- Angina
- Anemia associated with hemoglobinopathy
Table 2. Transfusion Reactions Reported to the South African Hemovigilance Program From 2000 to 2006
2006* 2005 2004 2003 2002 2001 2000 Total Acute hemolytic reactions 75 32 49 20 43 31 25 275 Incorrect blood product transfused 28 31 17 56 29 15 15 191 Delayed hemolytic reactions 0 1 1 4 7 3 9 25 Transfusion transmissible infections 1 4 4 3 5 5 0 22 Transfusion-related acute lung injury 3 2 2 0 0 0 0 7 Unclassified 11 1 2 1 9 17 1 42 Total 118 71 75 84 93 71 50 562 *The results for 2006 are not national results. The Western Province Blood Transfusion data were not included.
![]()
References
- Dhingra N. Blood safety in the developing world and WHO initiatives. Vox Sang 2002; 83(Suppl. 1): 173–7.
- World Health Organization. Developing a National Policy and Guidelines on the Clinical Use of Blood. Recommendations. World Health Organization: Geneva, 2004. Accessed at: http://www.who.int/bloodsafety/clinical_use/en/ (accessed 17 April 2007).
- Friedman MT, Ebrahim A. Adequacy of physician documentation of red blood cell transfusion and correlation with assessment of transfusion appropriateness. Arch Pathol Lab Med 2006; 130: 474–9.
- Dosunmu AO, Dada MO. Principles of blood transfusion service audit. Afr J Med Sci 2005; 34: 349–53.
- National Institute for Clinical Excellence. Principles for Best practice in Clinical Audit. Radcliff Medical Press Ltd.: Abingdon, 2002. Accessed at: http://www.nice.org.uk/ (accessed 17 April 2007).
- Wallis JP, Stainsby D, McClelland DB. Audit of red cell transfusion. Transfus Med 2002; 12: 1–9.
- Engelfriet CP, Reesink HW. Haemovigilance. Vox Sang 2006; 90: 207–41.
- Faber JC. Worldwide overview of existing haemovigilance systems. Transfus Apher Sci 2004; 31: 99–110.
- Arslan O. Haemovigilance in developing countries. Hematology 2005; 10(Suppl. 1): 79–81.
- American Association of Blood Banks. Standards for Blood Banks and Transfusion Services, 18th edn. AABB: Bethesda, MD, 1997.
- Kleinman S, Chiavetta J, Hindieh F, et al. The Surveillance and Epidemiology of Transfusions Working Group. Final Report. Public Health Agency of Canada: Ottawa, ON, 1999. http://www.phac-aspc.gc.ca/hcai-iamss/tti-it/pdf/setrep0299_e.pdf (accessed 17 April 2007).
- Kicklighter EJ, Klein HG. Hemolytic transfusion reactions. In: Linden JV, Bianco C (eds). Blood Safety And Surveillance, New York: Marcel Dekker, Inc, 2001.
- Nel TJ, Heyns A, Ndlovu Z, Asmal S. Haemovigilance Annual Report: Blood Transfusion Services of South Africa. South African Blood Transfusion Service: Bleomfontein, 2000–2005. Accessed at: http://www.sanbs.org.za (accessed 17 April 2007).
- Moshi G, Gulube S, Lutchman N, Ledwaba F. Haemovigilance Report 2006. South African Blood Transfusion Service: Bleomfontein, 2006.
Dr T. J. Nel, Department of Hematology and Cell Biology, University of the Free State, Bloemfontein, PO Box 28126, Danhof 9310, South Africa; Email: teresa.nel@pathcare.co.za
![]()
Teresa J. Nel, Department of Hematology and Cell Biology, University of the Free State, Bloemfontein, South Africa
Fonte: Medscape
Tags: Anestesia, Rianimazione




2 Responses to “Clinical Guidelines, Audits and Hemovigilance in Managing Blood Transfusion Needs”
Trackbacks/Pingbacks
Leave a Reply
You must be logged in to post a comment.