Margaret A. Pisani, MD, MPH; Terrence E. Murphy, PhD et al.
Crit Care Med. 2009;37(1):177-183. ©2009 Lippincott Williams & Wilkins – Posted 01/28/2009
Delirium is an acute disorder of attention and cognition with a reported prevalence of 78%-87% among older intensive care unit (ICU) patients.[1-5] Delirium leads to poor outcomes, including increased morbidity, mortality, nursing home placement, longer length of ICU and hospital stays, and costlier hospitalizations.[6-12] Very few studies have examined the duration of ICU delirium. Patients with longer ICU stays have increased risks for acquiring nosocomial infections and other ICU-related complications. Patients admitted to an ICU have many nonmodifiable risk factors for delirium such as age, severity of illness, and dementia. Use of psychoactive medications is a potentially modifiable risk factor for delirium.
Reported associations between benzodiazepines and opioids and delirium are inconsistent.[11, 13-18] The linkage between these medications and delirium has been examined through case reports and a small number of studies. The majority of studies did not ensure that the medication exposure preceded the development of delirium. We have recently shown that receipt of benzodiazepines before ICU admission was associated with delirium within the first 48 hrs of ICU admission.[19] Although researchers have examined the use of these medications as a risk factor for transitioning to delirium,[20] there are no studies examining associations between their use and delirium duration. Reducing delirium duration may improve patient outcomes by reducing days on mechanical ventilation, length of ICU and hospital stay, hospital morbidity related to falls and unnecessary restraint use.
The aim of this study is to examine the impact of benzodiazepines or opioids on the duration of ICU delirium. Our hypothesis is that after adjustment for potentially confounding factors, the use of benzodiazepines or opioids will prolong the first episode of ICU delirium.
Methods
Study participants were 304 consecutive patients ≥60 yrs admitted to the medical ICU of Yale-New Haven Hospital between September 5, 2002 and September 30, 2004. Yale-New Haven is a 900-bed university hospital with a 14-bed ICU. Of the 725 patients screened on admission, 318 were eligible. Reasons for noneligibility included admission for <24 hrs (n = 193), transfer from another ICU (n = 83), inability to communicate before admission (n = 52), no identifiable proxy (n = 56), and non-English speaking (n = 23). Of the 318 eligible patients, 309 (97%) were enrolled. Of the nine nonenrolled patients, eight were proxy refusals, and one patient refusal. Five patients were excluded from further analysis because of persistent stupor or coma. Informed consent was obtained from the patient and proxy according to procedures approved by the Institutional Review Board of Yale University.
Data Collection
Patients were critically ill; therefore, proxy respondents were used as the primary source of baseline information, as described previously.[21,22] The proxy interviews included questions on demographics, alcohol, smoking, and depression.[23] To evaluate the prevalence of dementia, we used the short form of the Informant Questionnaire on Cognitive Decline in the Elderly.[24,25] Baseline function was assessed with the Katz Activities of Daily Living Scale,[26] and Lawton’s Instrumental Activities of Daily Living Scale.[27]
Medical records were reviewed to obtain admitting diagnosis, Charlson Comorbidity Index,[28] the Acute Physiology and Chronic Health Evaluation Status score,[29] and laboratory markers of renal and liver dysfunction.
Medication Data
We recorded use of opioids (fentanyl and morphine), benzodiazepines (lorazepam and midazolam), and propofol on a daily basis. These medications are specified on the formulary for pain control and sedation in our ICU. During the enrollment period, there were no sedation or pain protocols available to physicians or nurses in our ICU. Our ICU does not use a daily awakening from sedation protocol. Drug choices were at the discretion of the treating physician. All routes of administration of medication were recorded as were the use of other psychoactive medications, including typical and atypical antipsychotics, seizure medications, and antidepressants. A research nurse blinded to the delirium assessments performed the chart reviews and recorded the medication data from both nursing flow sheets and the pharmacy’s electronic database.
Delirium Assessment
Delirium was assessed Monday through Friday using the Confusion Assessment Method-ICU, a validated instrument, with a sensitivity of 93%-100%, a specificity of 98%-100%, and high inter-rater reliability (kappa = 0.96).[1,30] Detailed information about the Confusion Assessment Method-ICU can be found at www.icudelirium.org. We supplemented the interviews using a validated chart review method daily.[4,31] Research nurses, who underwent training and interrater reliability testing for all key measures before study onset and at 6-month intervals, conducted study assessments. Inter-rater reliability was 100% between our two research nurses.
Delirium Duration
Our outcome variable, duration of ICU delirium, was the number of days in a first episode of delirium. Patients not having delirium during their ICU stay were assigned a count of zero. We defined an episode of ICU delirium as a sequence of consecutive days of delirium as assessed by the Confusion Assessment Method-ICU or chart review. In addition, if a nondelirium or coma day occurred between two days of delirium, the nondelirium day was included in the delirium episode. Each episode ended with 48 consecutive hours without delirium. A medication-related episode of delirium was defined as one occurring on the same day or within 48 hrs after receiving a benzodiazepine or opioid. Forty-eight hours was chosen based on the clinical judgment that medications received before 48 hrs were unlikely to contribute to the delirium episode. A nonmedication-related delirium episode was defined as delirium occurring without prior receipt of medications or >48 hrs after receiving benzodiazepines or opioids. Defining our outcome in this manner established a temporal relationship in which benzodiazepines or opioids were always received before or on the same day as the episode of delirium.
Predictor
Our primary predictor variable, receipt of a benzodiazepine or opioid during ICU stay, was dichotomous. We combined the receipt of benzodiazepines and opioids because 77% of the patients receiving these medications received both classes. Figure 1 displays the combinations of medications received by our study population.

Figure 1.Benzodiazepines and opioids used for sedation and pain control among study participants (n = 304). Benzodiazepines and Opioids includes: Lorazepam/Morphine, Lorazepam/Fentanyl, Midazolam/Morphine, Midazolam/Fentanyl, and Propofol/Fentanyl. Benzodiazepines only includes: Lorazepam only, Midazolam only, Lorazepam/Midazolam and Lorazepam/Midazolam/Propofol. Opioids only includes: Morphine only, Fentanyl only, and Morphine/Fentanyl.
Covariates and Interactions
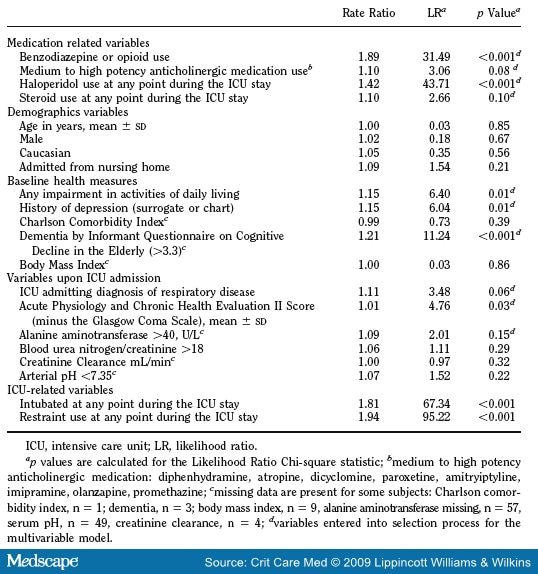
Candidate control variables were identified based on clinical experience and prior literature and are listed in Table 1 . Dementia was defined as an Informant Questionnaire on Cognitive Decline in the Elderly score >3.3; this cut-point achieves a balance between sensitivity and specificity for detecting dementia.[21,32,33] Patients were considered to have depression if the proxy respondent or chart review documented depression. Because our outcome of delirium was highly correlated with the Glasgow Coma Scale, we used the Acute Physiology and Chronic Health Evaluation Status II score minus the Glasgow Coma Scale.[1,11] Physiologic variables included abnormal liver function indicated by an ALT >40 U/L, dehydration indicated by a blood urea nitrogen/creatinine ratio >18, creatinine clearance, and acidemia indicated by an arterial pH <7.35. Other variables included receipt of other medium to high-potency anticholinergic medications and use of steroids.
Statistical Analysis
Descriptive statistics consist of counts and percentages for categorical variables, and means and standard deviations for continuous variables. Bivariate analyses of the associations between receipt of benzodiazepines or opioids and delirium duration, and between candidate control variables and delirium duration, were conducted using Poisson regression models containing a variable quantifying each study participant’s time at risk for occurrence or increase in delirium duration.[34] Poisson regression was used because our outcome was a count of days of delirium.
Benzodiazepine or opioid use was forced into all models used in the multivariable forward selection process seeking to identify potent control variables. Candidate control variables ( Table 4 ) were entered into the selection process if they had a likelihood ratio chi-square statistic of >1.64 corresponding to a p value <0.20. Correlations among explanatory variables were examined with Kendall’s tau-b correlation coefficient as part of the process of identifying control variables. All patients who required intubation received benzodiazepines or opioids while on mechanical ventilation, and so it was not possible to adjust for intubation in multivariable models.
Goodness of fit was assessed by residual analysis, deviance statistics, and sensitivity analysis. Final model coefficients were internally validated using bootstrapping.[35] The dementia covariate had missing values (n = 3). A complete case analysis was conducted because any bias introduced by this number of missing values was likely minimal. All statistical tests were two-tailed, with p < 0.05 indicating statistical significance. Analyses were performed with SAS statistical software version 9.1.3 (Cary, NC).
Results
Characteristics of the study population are presented in Table 1 . The mean age of the participants was 75 yrs and 47% were male. Of the 304 patients, 239 (79%) had delirium during their ICU stay. Seventy-two percent had delirium on their first day of ICU admission. The median duration of ICU delirium was 3 days, with a range of 1-33 days. One hundred thirteen patients had delirium until death or ICU discharge. Data on the receipt of benzodiazepines or opioids and delirium are presented in Table 2 .
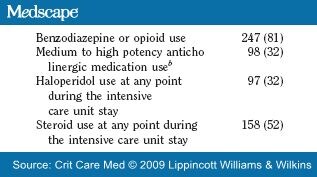
The drug data for this study are presented in Table 3 . The majority of patients received both classes of medications (benzodiazepines and opioids). Only 32 patients (11%) received opioids and 28 (9%) received benzodiazepines, exclusively. Twenty-one patients received propofol and a benzodiazepine and/or an opioid. Forty-two percent of our patients received a combination of three or more of these medications (Fig. 1). Thirty-two percent of the patients received haloperidol and 32% received other medium to high potency anticholinergic medications listed in Table 1 .
We examined indication for these medications. Of the 219 patients who received opioids, 100 had no pain score documented. Of the 119 patients with documented pain scores on their first day of receiving opioids, 86 (72%) had evidence of pain whereas 33 (28%) had no pain documented. Of the 215 patients who received benzodiazepines 213 had a level of consciousness assessed with a sedation scale on the first day they received medication. Of these latter 213 patients, 42 (20%) were agitated, 59 (28%) were lethargic, 50 (23%) had stupor or coma, and 62 (29%) were alert and calm. Seventy percent of patients who received haloperidol were agitated.
Bivariate analyses of the primary predictor and candidate control variables with the delirium duration outcome are reported in Table 4 . In addition to the receipt of benzodiazepines-opioids, several candidate control variables were significantly associated with delirium duration including impairment in the activities of daily living, depression, dementia, severity of illness, intubation, restraint use, and haloperidol use.
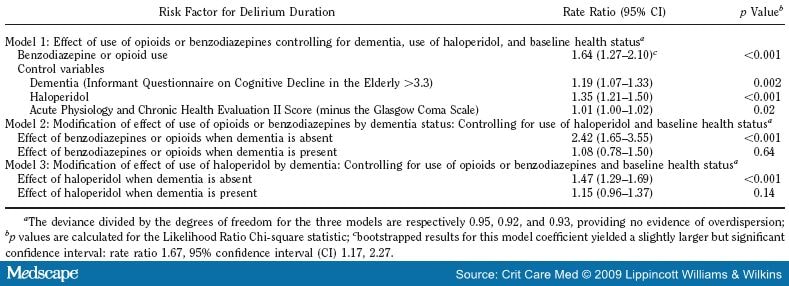
Table 5 presents our multivariable models of delirium duration. The top section of Table 5 reports effects of benzodiazepine-opioid use while controlling for baseline dementia, haloperidol, and health status. Use of a benzodiazepine or opioid shows statistically significant positive association with the duration of ICU delirium. Control variables for dementia, use of haloperidol, and Acute Physiology and Chronic Health Evaluation Score II also have positive significant associations with delirium duration. The middle section of Table 5 summarizes the modification of the effect of benzodiazepine-opioid use by dementia controlling for use of haloperidol and baseline health status (interaction term p value <0.01). For patients without dementia, there is a statistically significant, positive association between benzodiazepine-opioid use and delirium duration. Specifically, among the subgroup without dementia, there was a 142% increase in the rate of delirium for those receiving benzodiazepines or opioids as compared with persons without dementia not receiving these medications (rate ratio -2.42, 95% confidence interval 1.65-3.55). For patients with dementia this positive association is reduced and is not significant. There was no significant interaction between uses of benzodiazepine-opioid and haloperidol.
We also examined the association of benzodiazepine-opioid use with delirium duration in the 142 patients who were never intubated. In this group there was a nonsignificant trend toward increased delirium duration in the patients receiving benzodiazepines or opioids (rate ratio 1.18, 95% confidence interval 0.88-1.59, p = 0.26).
Discussion
In our cohort of older ICU patients, receipt of benzodiazepines or opioids was significantly associated with prolonged delirium duration even after adjusting for severity of illness, haloperidol use, and dementia. This is the first study to examine use of benzodiazepines and opioids and their association with duration of ICU delirium. Prior studies examining medications and delirium have used varying definitions of medication exposure. Some studies grouped all medications together, others examined only medications with anticholinergic properties, whereas others reviewed only individual classes of drugs such as antidepressants, benzodiazepines, opioids, and steroids.[11, 36-40] Reported associations between opioid use and delirium are inconsistent. Although one study in patients with cancer did show a significant impact of opioid use on the risk of delirium,[18] several studies have failed to identify a significant association.[41-48] Two previous ICU studies to examine opioid use and delirium found no significant association.[11,20] In a recent review of 11 studies examining medication and the delirium in hospitalized patients,[49] only four studies found a positive association between benzodiazepines and delirium. The magnitude of the association between benzodiazepine use and delirium varied depending on the dosage given and the properties of the medication, with short-acting benzodiazepines having lower risk.[12,40,47,50] In a large ICU cohort the use of sedatives and opiates did not differ in patients with or without delirium and their use increased the risk of delirium only when used to induce coma.[11] Because the majority of our participants (77%) received both benzodiazepines and opioids during their ICU stay, we have grouped them together. In this ICU-based study we demonstrate a positive association between their collective use and duration of delirium among older patients.
Although Kress et al[51] did not examine delirium, they demonstrated that daily interruption of sedation decreased duration of mechanical ventilation and length of ICU stay, without increasing adverse outcomes.[52,53] Their study is inconclusive regarding whether the reported improvement in ICU outcomes may be related to a reduction in the duration of delirium. Our ICU does not use a sedation interruption protocol so we could not examine the association with duration of delirium.
Thirty-two percent of our patients also received additional medium to high potency anticholinergic medications. We combined these medications into a group as the individual numbers were too small. Although these medications were of borderline significance in bivariate analysis (p = 0.08), they were not associated with delirium duration in our multivariable model. Although there is evidence that anticholinergic medications are linked to delirium in older, non-ICU patients, no studies have evaluated their use in an ICU population.[36,54] It may be that their contribution to duration of delirium is diminished in critically ill patients receiving benzodiazepines and opioids.
We included haloperidol as a control variable based on the clinical supposition that haloperidol may shorten delirium duration in patients receiving benzodiazepines or opioids. Our results did not support this idea. Haloperidol’s recommended status for treatment of delirium,[55-57] is based on limited data. There are no randomized placebo controlled studies in critically ill patients demonstrating association between haloperidol and improvement in symptoms or duration of delirium.[57] One study comparing haloperidol to olanzapine found that both medications reduced delirium symptoms.[57]
We hypothesize that haloperidol may treat symptoms of delirium (such as agitation) without reducing duration of delirium. We reviewed receipt of haloperidol and sedation status in our cohort and found that the majority of patients had delirium and 70% had agitation on the first day they received haloperidol. However, we do not have documentation on what prompted prescription of haloperidol to our study patients. Recent reports from the Federal Drug Agency have highlighted serious adverse effects of haloperidol when used off label in an ICU setting.[58] Given the definition of our outcome, delirium duration, which was designed to establish a temporal relationship with receipt of benzodiazepines and opioids, we cannot draw statistical inferences for haloperidol. This finding for haloperidol, however, raises interesting questions for future research directions, such as randomized controlled trials of delirium treatment in the ICU.
Neither benzodiazepine-opioid use nor haloperidol use was significantly associated with delirium duration among the subgroup of the study sample having dementia. This was likely because of a ceiling effect among patients with dementia who collectively spent close to 80% of their ICU days in a state of delirium.
The major innovation of our study is its examination of duration of delirium rather than occurrence. This is advantageous in an ICU study because so many patients have delirium on the first day of their ICU stay. A second strength is the firm establishment of a temporal ordering between receipt of medications and delirium to ensure their receipt before or concomitant with the first episode of delirium. We also examined potential confounders relevant to drug metabolism including liver function and creatinine clearance, allowing us to assess relevant patient factors which may impact the relationship between medications and delirium.
Our study is limited in that we included only older medical ICU patients from a single site. Further studies are needed to determine whether these results are generalizable to other settings. We did not examine benzodiazepines and opioids separately because only 28 participants received a benzodiazepine exclusively, 32 received an opioid exclusively, and all 21 receiving propofol also received a benzodiazepine and opioid. Our data reflect the common ICU practice of giving benzodiazepines and opioids together for sedation and pain control. Ideally, these drugs should be investigated separately as there may be a class effect. Our study examined the duration of ICU delirium but did not count delirium days occurring after ICU discharge. Because 27% of our patients had delirium at ICU discharge, implying their episode may have continued postdischarge, our measure of duration of delirium is conservative.
A notable limitation of this study was our inability to separate the effect of these medications from the consequences of intubation. We examined this potential confounder by comparing benzodiazepine or opioid use with delirium duration by intubation status. There was a nonsignificant trend toward increased delirium duration in the 141 nonintubated patients who received benzodiazepines or opioids (rate ratio 1.18, p = 0.26). Although a precise use of dosage was not feasible in our model it is likely that the intubated patients received a higher quantity of medications. Additional comparison revealed that intubated patients had higher Acute Physiology and Chronic Health Evaluation Status II scores, lower Pao2/Fio2 ratios and more days of medication. Although it is conceivable that the baseline differences in disease severity and total dose of medication account for much of the difference in the effect of interest between the intubated and nonintubated subgroups, it is not an issue that can be confirmed in these data set.
Whereas prevention of delirium is an ideal approach and an area of ongoing investigation, many older patients present to the ICU with delirium. Of the 239 delirious patients in our study, the majority were delirious on their day of admission (72%). Although prevention of delirium within the ICU is difficult, it may be possible to reduce delirium duration. In an unadjusted comparison, patients who received benzodiazepines or opioids had an average ICU delirium duration of 5.79 days for each week at risk, compared with 3.08 days for patients who did not receive benzodiazepines or opioids. Although there is no evidence that reducing delirium duration will lead to improved outcomes, there is evidence that delirium duration may be linked to adverse neuropsychological outcomes.[59] The impact of reducing delirium duration on outcomes needs to be investigated. Although optimal treatment of the underlying illness is of paramount importance, our data suggest that a reduction in the administration of benzodiazepines and opioids may also be beneficial.
Future studies are needed to determine the optimal medications for sedation, pain and agitation in critically ill patients. Eighty-one percent of patients received benzodiazepines when their Richmond Agitation Sedation Scale score indicated that the patient was alert and calm, lethargic, or had stupor/coma. This implies that we may be over sedating the patients. Until there is further evidence to guide treatment, we recommend regulation of benzodiazepine and opioid use through implementation of sedation protocols, daily interruption of sedation, and frequent reassessment of sedation goals.[51] Studies are needed to determine whether lowering dosage of benzodiazepines or opioids reduces duration of delirium and whether shorter duration results in better patient outcomes.
Table 1. Characteristics of the Study Population (n = 304)
Table 2. Scenarios for Delirium and Receipt of Benzodiazepine or Opioid Medications (n = 304)
Table 3. Drug Data for the Study Population (n = 304)
Table 4. Bivariate Analysis of Variables for Delirium Duration Outcome (n = 304)
Table 5. Multivariable Models for Delirium Duration (n = 304)
![]()
References
|
We acknowledge the contributions of Peter Charpentier for database development, Wanda Carr for data entry; Karen Wu and Andrea Benjamin for enrolling participants and interviewing family members, and Susan Ardito for her careful review of the manuscript. We thank the families, nurses, and physicians in the Yale Medical Intensive Care Unit, whose cooperation and participation made this study possible.
This work was supported, in part, by ID# CG-002-N from the American Lung Association and Connecticut Thoracic Society, by P30AG21342 from Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine, from the T. Franklin Williams Geriatric Development Initiative through The CHEST Foundation, ASP, Hartford Foundation. Dr. Pisani is a recipient of a NIH K23 Mentored Career Development Award (K23 AG 23023-01A1). Dr. Inouye is supported in part by Grants #R21AG025193 and #K24AG000949 from the National Institute on Aging.
The authors have not disclosed any potential conflicts of interest.
Dr. Inouye holds the Milton and Shirley F. Levy Family Chair.
Author Contributions: Dr. Pisani had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; Study concept and design, Pisani, Araujo, Inouye; Analysis and interpretation of the data, Pisani, Murphy, VanNess, Araujo, Inouye; Drafting of the manuscript, Pisani, Murphy, VanNess; Critical Revision of the manuscript for important intellectual content, Pisani, Murphy, VanNess, Araujo, Inouye; Statistical analysis, Murphy, VanNess; Obtained funding, Pisani, Inouye; Administrative and technical support, Araujo; Study supervision, Pisani.
For information regarding this article, E-mail: margaret.pisani@yale.edu
![]()
Tags: News, Rianimazione






Leave a Reply
You must be logged in to post a comment.