Ian S deSouza, MD, Assistant Professor, Department of Emergency Medicine, Kings County Hospital/SUNY Downstate Medical Centers
Che’ Damon Ward, MD, Staff Physician, Department of Emergency Medicine, State University of New York Health Science Center at Brooklyn
Updated: Dec 17, 2008
Introduction
Background
Ventricular tachycardia (VT) is a tachydysrhythmia originating from a ventricular ectopic focus, characterized by a rate typically greater than 120 beats per minute with wide QRS complexes. VT may be monomorphic (originating from a single focus with identical QRS complexes) or polymorphic (may appear as an irregular rhythm, with varying QRS amplitudes and morphology). Nonsustained VT is defined as a run of tachycardia of less than 30 seconds duration; longer runs are considered sustained VT.
No absolute ECG criteria exist for establishing the presence of VT. However, several factors suggest VT, including the following:
* Rate greater than 120 beats per minute (usually 150-200)
* Wide QRS complexes (>140 ms)
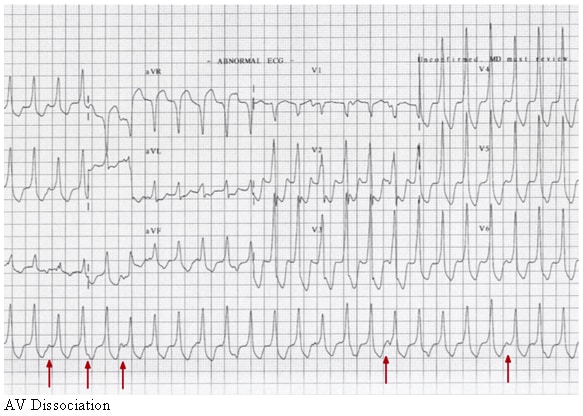
* Presence of atrioventricular (AV) dissociation
* Fusion beats
* Capture beats
Ventricular tachycardia may develop without hemodynamic deterioration. Nevertheless, it often causes severe hemodynamic compromise and may deteriorate rapidly into ventricular fibrillation (VF). This tachydysrhythmia must be addressed swiftly to avoid morbidity or mortality.
Pathophysiology
Ventricular tachycardia (VT) usually is a consequence of structural or ischemic heart disease, with breakdown of normal conduction patterns. Increased automaticity (which tends to favor ectopic foci) or activation of reentrant pathways in the myocardium can exist. Electrolyte disturbances, ischemia, and sympathomimetics may increase the likelihood of VT in the susceptible heart. AV dissociation is present in approximately half of VT episodes.1 However, to confuse the diagnosis, retrograde ventriculoatrial conduction may occur, which can generate an ECG tracing similar to paroxysmal supraventricular tachycardia (PSVT) with aberrant conduction.
When present, AV dissociation is a hallmark characteristic of VT. This occurs when the sinus node is depolarizing the atria at its normal rate, which is usually slower than the pathologic ventricular rate. P waves can be visualized at times in between or embedded in the QRS complexes, but the two have their own independent rate. Fusion beats and capture beats can occur in the presence of VT depending on the refractory period of the AV node and the timing of ventricular and atrial depolarizations, respectively.
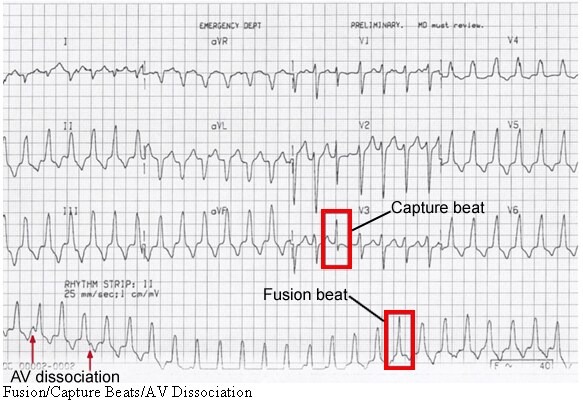
Fusion beats have a mixed morphology, due to normal AV/His bundle conduction occurring simultaneously with abnormal (wide complex QRS) ventricular depolarization. Thus, an impulse travels from the AV node through the normal conduction pathway (a narrow QRS), and the competing impulse originates from the abnormal ectopic ventricular focus (a wide QRS). The two converge leading to a mixed (fused) QRS.
A capture beat occurs when an atrial impulse arrives at the AV node at a “fortuitous” time when it has just recovered from its refractory period. The timing has to be just right as the AV node is frequently in its refractory state due to retrograde conduction from the rapid ventricular rhythm. Conduction thus proceeds normally through the AV node/His-Purkinje system leading to a narrow QRS complex.
AV dissociation.
Fusion, capture beats, and AV dissociation.
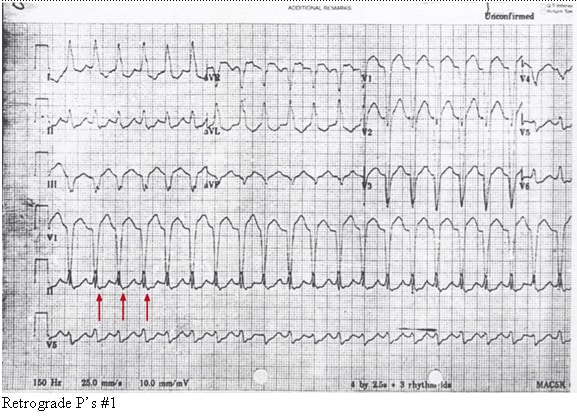
Retrograde conduction can also exist from the ventricles to the atria via the AV node. This can lead to an apparent 1:1 correlation between the wide QRS complex and the P wave. This is seen in two different types of supraventricular tachycardias: (1) antidromic AV reentrant tachycardia (AVRT) and (2) AV nodal reentrant tachycardia with aberrant conduction (AVNRT).
Antidromic AVRT occurs due to the properties of the atrioventricular node and the accessory conduction pathway. Forward, or anterograde, conduction through the accessory pathway occurs, leading to a widened QRS complex with backward, or retrograde, conduction through the AV node causing retrograde P waves. AVNRT with aberrant conduction occurs when a reentrant circuit in the AV node propagates impulses both anterograde through an abnormal His-Purkinje system (thus the wide QRS) and also retrograde throughout the atria creating retrograde P waves. These two rhythms may be indistinguishable from VT with 1:1 ventriculo-atrial conduction.
Retrograde P’s #1.
The most common cause of monomorphic sustained VT is a prior MI with ventricular myocardial scar formation. The presence of myocardial fibrosis is a substrate for slow conduction pathways and associated reentry mechanisms. Nonsustained VT and ectopy is more commonly associated with acute myocardial ischemia.
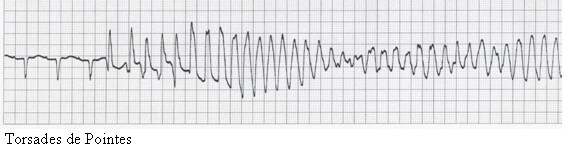
A distinctive variant of VT is torsade de pointes, with its unusual shifting-axis QRS complexes that appear (on ECG) as if the heart is rotating upon an axis. It typically occurs during sinus rhythm and in the presence of drugs or conditions that prolong the QT interval (eg, type 1A antiarrhythmics, hypomagnesemia, droperidol). The dysrhythmia may occur in either the presence or the absence of myocardial ischemia or infarction.
Torsades de pointes.
A second variant of VT is accelerated idioventricular rhythm. Sometimes termed slow ventricular tachycardia, this dysrhythmia presents with a rate of 60-100 beats per minute. It typically occurs with underlying heart disease (ischemic or structural), is transient, and only rarely is associated with hemodynamic compromise or collapse. Treatment of the dysrhythmia itself usually is not required unless hemodynamic impairment develops.
Due to advances in molecular biology, a number of inherited dysrhythmic disorders with a propensity toward VT have been described. Brugada syndrome, congenital long and short QT syndrome, and catecholaminergic-sensitive VT have complex inheritance patterns. Mechanistically, each disorder is characterized by imbalanced ion transport across the cardiac cellular membrane, which leads to abnormalities in cardiac repolarization, thus increasing risk of dysrhythmia. These syndromes have all been linked to sudden cardiac death. Patients with these disorders are being managed with a combination of genetic typing, antidysrhythmic medications, lifestyle modification, and implantable cardioverter-defibrillators (ICD).
Frequency
United States
Nonsustained, short runs of VT are frequently observed dysrhythmias, but sustained monomorphic VT is uncommon in the ED setting due to aggressive treatment of myocardial ischemia.
International
VT and coronary artery disease are common throughout most of the developed world. In developing countries, VT and other heart diseases are relatively less common.
Mortality/Morbidity
- Morbidity and mortality in VT arise principally from spontaneous degeneration into the more malignant ventricular defibrillation.
- Even without such degeneration, VT can also produce congestive heart failure and hemodynamic compromise, with subsequent morbidity and mortality.
Sex
- Currently, most patients presenting with VT are men.
- As coronary artery disease (CAD) becomes more common in women, it seems certain that the incidence of VT in women will increase.
Age
- VT is unusual among pediatric patients, although when present there is associated congenital heart disease, or it occurs in the postoperative cardiac setting. Tachydysrhythmias in this population generally are PSVT.
- VT incidence rates peak in the middle decades of life, following the incidence of structural heart disease.
Clinical
History
Most patients with ventricular tachycardia (VT) present to the ED with symptoms of either ischemia or hemodynamic compromise. These may include the following:
- Chest discomfort
- Dyspnea
- Diaphoresis
- Nausea
- Palpitations
- Anxiety or feeling of “impending doom”
- Syncope and presyncope
Physical
Besides tachycardia, findings generally reflect the degree of hemodynamic instability.
- Signs of congestive heart failure (CHF)
- Hypotension
- Hypoxemia
- Jugular venous distention
- Rales from pulmonary edema
- Mental status changes
- Anxiety
- Agitation
- Lethargy
- Coma
- Subtle signs of AV dissociation
- Irregular cannon a waves in the jugular pulse
- Variable intensity of the first heart sound
- Beat-to-beat changes in systolic blood pressure
Causes
As noted above, VT generally is a consequence of ischemic or structural heart disease or electrolyte deficiencies (eg, hypokalemia, hypocalcemia, hypomagnesia). It can also be triggered by the following:
- Use of sympathomimetic agents (from relatively benign caffeine to more potent agents such as methamphetamine or cocaine)
- Drugs that prolong the QT complex (eg, type 1A antiarrhythmics, droperidol and related phenothiazines) – These particularly cause torsade de pointes.
- Rheumatologic disorders that affect the myocardium systemic such as systemic lupus erythematosus and rheumatoid arthritis
- Other structural congenital disorders such as right ventricular dysplasia and tetralogy of Fallot
- Digitalis toxicity – This can lead to biventricular tachycardia.
- Channelopathies as mentioned above
Differential Diagnoses
| Atrial Fibrillation | Hypomagnesemia |
| Atrial Flutter | Myocardial Infarction |
| Automatic External Defibrillation | Pacemaker and Automatic Internal Cardiac Defibrillator |
| Congestive Heart Failure and Pulmonary Edema | Premature Ventricular Contraction |
| Hypocalcemia | Torsade de Pointes |
| Hypokalemia | Ventricular Fibrillation |
Other Problems to Be Considered
Supraventricular tachycardia (SVT) with aberrancy
Sudden cardiac death
Workup
Laboratory Studies
- When the patient presents with symptoms of frank hemodynamic compromise, defer laboratory tests until electrical cardioversion or defibrillation is performed and the patient is stabilized.
- Assess electrolyte levels of all patients with ventricular tachycardia (VT), including serum calcium, magnesium, and phosphate levels. Ionized calcium levels are preferred over total serum calcium level. Hypokalemia, hypomagnesemia, and hypocalcemia may predispose patients to either monomorphic VT or torsade de pointes.
- Obtain, when appropriate, levels of therapeutic drugs (eg, digoxin).
- Evaluate for myocardial ischemia or infarction with serum cardiac troponin I or T levels or other cardiac markers.
Imaging Studies
- Chest radiography is indicated if symptoms suggest the possibility of congestive heart failure (CHF) or other cardiopulmonary pathology as contributing factors.
Other Tests
- ECG is the diagnostic tool of choice for confirming the presence of VT. Simultaneous 3-channel recordings and 12-lead tracings are more helpful than rhythm strips to analyze such dysrhythmias.
- Complexes of atypical morphology often are difficult to interpret. Such tachycardias could be paroxysmal supraventricular tachycardia (PSVT) with aberrant conduction. If the patient is unstable, or differentiation between VT and SVT is uncertain, treat rhythm as VT. Recall that the vast majority of patients with wide-complex regular tachycardias will have VT. Recognize that some therapies for PSVT (eg, verapamil) can be lethal when used in VT.
- ECG criteria that support VT over SVT include AV dissociation, fusion beats at the initiation of the arrhythmia, QRS duration over 140 ms, and RS pattern in V1, frontal QRS axis between 180 and 270 degrees, and positive or negative concordance across the precordial leads.2 Patients with underlying structural or ischemic heart disease are more likely to have VT than SVT.
- ECG criteria that support SVT over VT include a right bundle branch block (RBBB) pattern when present in the native sinus rhythm, varying bundle branch block, an R or qR pattern in V1, or an ectopic P wave preceding the dysrhythmia.
Treatment
Prehospital Care
EMTs and paramedics may be called upon to provide cardioversion/defibrillation in the field if they have sufficient training and if appropriate protocols exist.
- Rapid transport to an ED is essential.
- EMS personnel must pay adequate attention to the primary survey and address the ABCs as necessary. Beyond those steps, vascular access, supplemental oxygen, and ECG rhythm strip monitoring are all important but should not delay rapid transport to the ED for definitive care.
Emergency Department Care
During the initial assessment, once real-time cardiac monitoring or 12-lead ECG has established VT as the diagnosis, determine if the VT is stable or unstable as the ABCs are reassessed in the primary survey.
- Pulseless VT
- Pulseless VT, in contrast to other unstable VT rhythms, is treated with immediate defibrillation. High-energy, unsynchronized energy should be used. The initial shock dose on a biphasic defibrillator is 200 J followed by an equal or higher shock dosage for subsequent shocks. If a monophasic defibrillator is used, the initial and subsequent shock dosage should be 360 J. Shock administration should be followed by immediate CPR, airway management, supplemental oxygen, vascular access, vasopressor agents, and then consideration of antidysrhythmic administration.
- Vasopressors can include epinephrine at 1 mg IV given every 3-5 minutes, or in lieu of epinephrine, vasopressin 40 U IV as a one-time dose.
- Advanced cardiac life support (ACLS) drug therapy guidelines now recommend the use of amiodarone or lidocaine as the first-line adjunctive antidysrhythmic treatment of shock-resistant pulseless VT.
- Unstable VT
- Unstable VT is characterized by signs/symptoms of insufficient oxygen delivery to vital organs such as chest pain, dyspnea, hypotension, or altered level of consciousness, indicating that rate and stroke volume are not providing adequate cardiac output.
- In this situation, the dysrhythmia should be immediately treated with synchronized cardioversion, usually at a starting energy dose of 100 J.
- In contrast, polymorphic VT is treated with immediate defibrillation as the defibrillator may have difficulty recognizing the varying QRS complexes and thus synchronizing the delivery of energy.
- Antidysrhythmic therapy as outlined above for shock-resistant pulseless VT may be administered to those with shock-resistant unstable VT.
- Stable VT
-
- Stable VT usually denotes monomorphic VT with adequate vital end-organ perfusion. These patients do not experience signs/symptoms of hemodynamic compromise.
- Stable VT can be treated with amiodarone, procainamide, or sotalol. Some evidence indicates that procainamide may be more effective than amiodarone in the treatment of stable VT.3, 4 In situations involving torsade de pointes, magnesium sulfate may be effective if there is a long QT interval at baseline.
- Consider synchronized cardioversion early if medical therapy fails to stabilize the rhythm. Initial monophasic shock energy should be 100 J, followed by higher shock energies if the response is inadequate.
Consultations
Following initial treatment and stabilization, patients with ventricular tachycardia (VT) generally should be referred to a cardiologist for admission to a monitored bed, further studies, and definitive management.
Only rarely will a patient with stable, recurrent episodes of VT have his or her dysrhythmia treated in the ED and be discharged with appropriate follow-up care. This decision must be made in consultation with a cardiologist.
Medication
The mainstays of treatment for clinically stable VT are the various antidysrhythmic drugs.
Antiarrhythmics
These agents alter the electrophysiologic mechanisms responsible for arrhythmia.
Amiodarone (Cordarone)
Newest of the antiarrhythmics used in treating VT, generally is considered a class III antiarrhythmic, yet it has pharmacologic characteristics of all 4 classes.
Now is considered a class I intervention by the American College of Cardiology’s practice guidelines for managing acute MI. DOC in treatment of refractory, hemodynamically unstable VT. Prehospital studies suggest amiodarone is safe for use in prehospital setting, and its adoption in the new ACLS guidelines will increasingly lead EMS authorities to adopt it as their first-line antiarrhythmic. This change already is well underway in Europe.
Dosing
Adult
150 mg IV, infused over 10 min, then 1 mg/min constant infusion for 6 h, then maintenance infusion at 0.5 mg/min
Pediatric
Not established
Interactions
Increases effect and blood levels of theophylline, quinidine, procainamide, phenytoin, methotrexate, flecainide, digoxin, cyclosporine, beta-blockers, and anticoagulants; ritonavir, sparfloxacin, and disopyramide increase cardiotoxicity; coadministration with calcium channel blockers may cause additive effects, further decreasing myocardial contractility; cimetidine may increase amiodarone levels
Contraindications
Documented hypersensitivity; systemic lupus erythematosus, digitalis-induced arrhythmias, complete heart block, or second- or third-degree heart block if a pacemaker is not in place; torsade de pointes
Precautions
Pregnancy
D – Fetal risk shown in humans; use only if benefits outweigh risk to fetus
Precautions
Hypotension (most common adverse effect), bradycardia, and AV block may occur; elevation of serum hepatic enzyme levels is common in VT; monitor patients carefully
Lidocaine (Xylocaine, Nervocaine, LidoPen, Duo-Trach)
Class IB antiarrhythmics stabilize cell membranes, blunts phase 0 of the action potential, and shortens repolarization. Their net effect is to decrease firing of ectopic foci to allow a normal rhythm to reassert itself.
However, amiodarone appears to be replacing lidocaine as the drug of choice.
Dosing
Adult
1-1.5 mg/kg IV push, followed by 0.5-0.75 mg/kg IV push, not to exceed 3 mg/kg
Start continuous 1-4 mg/min infusion after arrhythmia is suppressed
Pediatric
1 mg/kg IV/ET/IO loading dose; may repeat twice at 10- 15-min intervals prn
Following loading dose, start continuous IV infusion 20-50 mcg/kg/min
Interactions
Coadministration with cimetidine or beta-blockers increases toxicity of lidocaine; coadministration with procainamide and tocainide may result in additive cardiodepressant action; may increase effects of succinylcholine
Contraindications
Documented hypersensitivity; Adams-Stokes syndrome and Wolff-Parkinson-White syndrome; severe sinoatrial, AV, or intraventricular block, if artificial pacemaker is not in place
Precautions
Pregnancy
B – Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Use a solution without preservatives; caution in heart failure, hepatic disease, hypoxia, hypovolemia or shock, respiratory-depression, and bradycardia; may increase risk of CNS and cardiac adverse effects in elderly patients; high plasma concentrations can cause seizures, heart block, and AV conduction abnormalities
Procainamide (Procanbid, Pronestyl)
Class IA antiarrhythmic, slows down phase 4 (diastolic) depolarization, decreases automaticity, and slows intraventricular conduction.
Second-line therapy used for VT refractory to defibrillation, epinephrine, and lidocaine, it increases refractory period of atria and ventricles. Myocardial excitability is reduced by an increase in threshold for excitation and inhibition of ectopic pacemaker activity.
Dosing
Adult
20-30 mg/min IV at continued infusion rates until either arrhythmia is suppressed, patient becomes hypotensive, QRS widens 50% above baseline, or a maximum dose of 17 mg/kg is administered
Once arrhythmia is suppressed, may infuse at a continuous rate of 1-4 mg/min
Pediatric
Not established; the following doses have been suggested:
15-50 mg/kg/d PO divided q3-6h; not to exceed 4 g/d
20-30 mg/kg/d IM divided q4-6h; not to exceed 4 g/d
3-6 mg/kg/dose IV infused over 5 min
Maintenance: 20-80 mcg/kg/min administered as continuous infusion; not to exceed 100 mg/dose or 2 g/d
Interactions
Can expect increased levels of procainamide metabolite, NAPA, in patients taking cimetidine, ranitidine, beta-blockers, amiodarone, trimethoprim, and quinidine; may increase effect of skeletal muscle relaxants, lidocaine, and neuromuscular blockers; ofloxacin inhibits tubular secretion of procainamide and may significantly increase its blood levels; when taken concurrently with sparfloxacin, may increase risk of cardiotoxicity
Contraindications
Documented hypersensitivity; complete heart block or second- or third-degree heart block, if pacemaker is not in place; torsade de pointes; documented hypersensitivity; systemic lupus erythematosus
Precautions
Pregnancy
C – Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Monitor patients for hypotension; plasma concentration of procainamide and its active metabolite, NAPA, may be increased in renal failure; high or toxic concentrations may induce AV block or abnormal automaticity; use caution in patients with complete AV block, digitalis intoxication, organic heart disease, renal disease, or hepatic insufficiency
Sotalol (Betapace)
Class III antiarrhythmic agent, which blocks potassium channels, prolongs action potential duration (APD) and lengthens QT interval. Noncardiac selective beta-adrenergic blocker.
Dosing
Adult
0.2 mg/kg to 1.5 mg/kg IV over 5 min
80 mg PO bid and increase dose gradually q2-3d to 240-320 mg/d
Pediatric
200 mg/m2/24 h up to 80 mg/dose divided bid maximum
Interactions
Aluminum salts, barbiturates, NSAIDs, penicillins, calcium salts, cholestyramine, and rifampin may decrease bioavailability and plasma levels, possibly resulting in decreased pharmacologic effect; cardiotoxicity of sotalol may increase when administered concurrently with sparfloxacin, calcium channel blockers, quinidine, flecainide, and contraceptives; toxicity of sotalol increases when administered concurrently with digoxin, flecainide, acetaminophen, clonidine, epinephrine, nifedipine, prazosin, haloperidol, phenothiazines, and catecholamine-depleting agents
Contraindications
Documented hypersensitivity; sinus bradycardia, second- and third-degree AV block
Precautions
Pregnancy
B – Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Beta-adrenergic blockade may decrease signs and symptoms of acute hypoglycemia and clinical signs of hyperthyroidism; abrupt withdrawal may exacerbate symptoms of hyperthyroidism, including thyroid storm; withdraw drug slowly and monitor patient closely; caution in hypokalemia, peripheral vascular disease, hypomagnesemia, congestive heart failure, and congestive heart failure
Electrolytes
These agents are considered therapeutic alternatives for refractory VT. Patients with persistent or recurrent VT following antiarrhythmic administration should be assessed for underlying electrolyte abnormalities as a cause for their refractory dysrhythmia. Some electrolyte abnormalities associated with VF include hyperkalemia, hypokalemia, and hypomagnesemia. Magnesium sulfate, calcium chloride, and sodium bicarbonate are used in VT secondary to other medications. Magnesium sulfate acts as an antiarrhythmic agent. Sodium bicarbonate is used as an alkalinizing agent, and calcium chloride is used to treat VT caused by hyperkalemia.
Magnesium sulfate (Magnesium)
DOC for torsade de pointes, it also may be useful to treat conventional VT, especially where hypomagnesemia is confirmed.
When treating with magnesium sulfate, monitor for hypermagnesemia since an overdose can cause cardiorespiratory collapse and paralysis.
Dosing
Adult
1-2 g diluted in 100 mL of D5W over 1-2 min for refractory VT and known or suspected hypomagnesemia (Mg+2 <1.4 mEq/L); not to exceed 30-40 g/d; maximum rate of infusion for maintenance not to exceed 1-2 g/h
Pediatric
Not established:
Suggested dose: 25-50 mg/kg q4-6h for 3-4 doses; maximum single dose of 2 g also may be administered and repeated if hypomagnesemia persists
Interactions
Concurrent use with nifedipine may cause hypotension and neuromuscular blockade; may increase neuromuscular blockade observed with aminoglycosides and other agents causing neuromuscular antagonism; may increase CNS depressant effects
Contraindications
Documented hypersensitivity; heart block; Addison disease; myocardial damage; severe hepatitis
Precautions
Pregnancy
A – Fetal risk not revealed in controlled studies in humans
Precautions
Monitor for hypotension and follow DTRs; if depressed DTRs observed, modify or halt dosage; may lead to heart block in digitalized patients; renal impairment may lead to accumulation and toxicity
Sodium bicarbonate (Neut)
Used only when patient is diagnosed with bicarbonate-responsive acidosis with pH ≤7.0, hyperkalemia, tricyclic antidepressant or phenobarbital overdose. Routine use is not recommended.
Dosing
Adult
Initial dose: 1 mEq/kg
Maintenance dose: 0.5 mEq/kg q10min or as indicated by ABGs
Pediatric
0.5-1 mEq/kg repeated q10min or as indicated by ABGs; rate of infusion not to exceed 10 mEq/min
Interactions
Urinary alkalinization, induced by increased sodium bicarbonate concentrations, may cause decreased levels of lithium, tetracyclines, chlorpropamide, methotrexate, and salicylates; conversely, use increases levels of amphetamines, anorexiants, mecamylamine, ephedrine, pseudoephedrine, flecainide, quinidine, and quinine
Contraindications
Patients with alkalosis, hypernatremia, hypocalcemia, severe pulmonary edema, and unknown abdominal pain
Precautions
Pregnancy
C – Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Only to be used to treat documented metabolic acidosis and hyperkalemia-induced cardiac arrest; can cause alkalosis, decreased plasma potassium, hypocalcemia, and hypernatremia; caution in electrolyte imbalances such as patients with CHF, cirrhosis, edema, corticosteroid use, or renal failure; when administering, should avoid extravasation since can cause tissue necrosis
Calcium chloride
Useful to treat hyperkalemia, hypocalcemia, or calcium channel blocker toxicity, it moderates nerve and muscle performance by regulating action potential excitation threshold.
Dosing
Adult
Known or suspected hyperkalemia (K+ >6 mEq/L): 2-4 mg/kg (10% solution) IV
Pediatric
0.2 mL/kg of IV (10% solution)
Interactions
Coadministration with digoxin may cause arrhythmias; with thiazides, may induce hypercalcemia; may antagonize effects of calcium channel blockers, atenolol, and sodium polystyrene sulfonate
Contraindications
VF not associated with hyperkalemia; digitalis toxicity; hypercalcemia; renal insufficiency; cardiac disease
Precautions
Pregnancy
C – Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Administer slowly (not to exceed 0.5-1 mL/min) to avoid extravasation; hypercalcemia may occur in renal failure
Vasopressor
These agents augment both coronary and cerebral blood flow present during low flow state associated with CPR.
Epinephrine (Adrenalin, Sus-Phrine, EpiPen)
Considered the single most useful drug in cardiac arrest, although it has never been shown to affect mortality.
Dosing
Adult
1 mg (10 mL of 1:10,000 solution) IV push q3-5min
ET administration requires 2-2.5 times IV dose
Pediatric
0.01 mg/kg or 0.3 mg/m2 SC (repeat q4h or more frequently prn)
Interactions
Increases toxicity of beta- and alpha-blocking agents and of halogenated inhalational anesthetics
Contraindications
Documented hypersensitivity; cardiac arrhythmias or angle-closure glaucoma; local anesthesia in areas such as fingers or toes because vasoconstriction may produce sloughing of tissue; do not use during labor (may delay second stage of labor)
Precautions
Pregnancy
C – Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Caution in elderly patients, prostatic hypertrophy, hypertension, cardiovascular disease, diabetes mellitus, hyperthyroidism, and cerebrovascular insufficiency; rapid IV infusions may cause death from cerebrovascular hemorrhage or cardiac arrhythmias
Vasopressin
May improve vital organ blood flow, cerebral oxygen delivery, ability to be resuscitated, and neurologic recovery.
Dosing
Adult
40 U IV single dose
Pediatric
Not established
Interactions
Lithium, epinephrine, demeclocycline, heparin, and alcohol may decrease effects; chlorpropamide, urea, fludrocortisone, and carbamazepine may potentiate effects
Contraindications
Documented hypersensitivity; coronary artery disease
Precautions
Pregnancy
C – Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Caution in cardiovascular disease, seizure disorders, nitrogen retention, asthma, or migraine; excessive doses may result in hyponatremia
Follow-up
Further Inpatient Care
- Admit patients with ventricular tachycardia (VT) to monitored settings as a precaution against recurrent tachydysrhythmia, to facilitate workup for ischemic heart disease, and to allow timely evaluation for definitive electrophysiologic or device therapy.
- In the past, long-term antidysrhythmic medical therapy was used for suppression of VT. However, several subsets of patients with VT do poorly under such an approach, with frequent recurrence of VT. As a result, cardiologists are increasingly making use of interventional therapy, with devices and procedures designed to abort VT or to remove the dysrhythmogenic foci in the heart. Such interventions include the following:
- Prophylactic implantation of ICDs
- Catheter-directed radiofrequency ablation of aberrant conduction pathways
- In a reported case in the United Kingdom, a patient whose VT was refractory even to ICD prophylaxis underwent bilateral thoracoscopic cervical sympathectomy, which successfully stopped the recurrent VT.5
Complications
- Patients with VT may suffer congestive heart failure (CHF) and its attendant morbidity as a result of hemodynamic compromise.
- VT may deteriorate to ventricular fibrillation (VF).
- Consider all patients with VT to have active myocardial ischemia, which should be sought and treated aggressively.
Prognosis
- If treated rapidly, VT generally has a favorable short-term outcome.
- Long-term prognosis depends upon the underlying cardiac disease.
Patient Education
- For excellent patient education resources, visit eMedicine’s Public Health Center. Also, see eMedicine’s patient education article Cardiopulmonary Resuscitation (CPR).
Miscellaneous
Medicolegal Pitfalls
- Failure to promptly treat hemodynamically compromised patients in a misguided effort to first secure a definite diagnosis
- Use of verapamil in a wide-complex tachycardia
- Failure to treat underlying diseases or conditions that may have precipitated the tachydysrhythmia
- Failure to aggressively assess the patient for myocardial ischemia after initial stabilization
Multimedia
Media file 1: AV dissociation.
Media file 2: Fusion, capture beats, and AV dissociation.
Media file 3: Retrograde P’s #1.
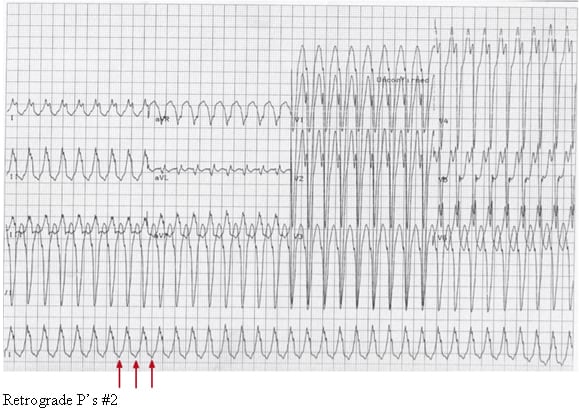
Media file 4: Retrograde P’s #2.
Media file 5: Torsades de pointes.
References
- Wellens HJ, Bar FW, Lie KI. The value of the electrocardiogram in the differential diagnosis of a tachycardia with a widened QRS complex. Am J Med. Jan 1978;64(1):27-33. [Medline].
- Akhtar M, Shenasa M, Jazayeri M, et al. Wide QRS complex tachycardia. Reappraisal of a common clinical problem. Ann Intern Med. Dec 1 1988;109(11):905-12. [Medline].
- Marill KA, deSouza IS, Nishijima DK, et al. Amiodarone is poorly effective for the acute termination of ventricular tachycardia. Ann Emerg Med. Mar 2006;47(3):217-24. [Medline].
- Tomlinson DR, Cherian P, Betts TR, et al. Intravenous amiodarone for the pharmacological termination of haemodynamically-tolerated sustained ventricular tachycardia: is bolus dose amiodarone an appropriate first-line treatment?. Emerg Med J. Jan 2008;25(1):15-8. [Medline].
- Turley AJ, Thambyrajah J, Harcombe AA. Bilateral thoracoscopic cervical sympathectomy for the treatment of recurrent polymorphic ventricular tachycardia. Heart. Jan 2005;91(1):15-7. [Medline].
- American Heart Association. Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 6: advanced cardiovascular life support: section 1: Introduction to ACLS 2000: overview of recommended changes in ACLS from the guidelines 2000 conference. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. Aug 22 2000;102(8 Suppl):I86-9. [Medline].
- Brennan TD, Haas GJ. The role of prophylactic implantable cardioverter defibrillators in heart failure: recent trials usher in a new era of device therapy. Curr Heart Fail Rep. Mar 2005;2(1):40-5. [Medline].
- Francis J, Sankar V, Nair VK, et al. Catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. May 2005;2(5):550-4. [Medline].
- Hoffman JR, Votey SR. Tachyarrhythmias. In: The Clinical Practice of Emergency Medicine. 2nd ed. 1996:605.
- Hunter R. Ventricular tachycardia following naloxone administration in an illicit drug misuse. J Clin Forensic Med. Aug 2005;12(4):218-9. [Medline].
- Kliegel A, Eisenburger P, Sterz F, et al. Survivors of ventricular tachyarrhythmias due to a transient or reversible disorder have a high recurrence rate of lethal cardiac events. Resuscitation. Sep 2002;54(3):237-43. [Medline].
- Kudenchuk PJ, Cobb LA, Copass MK, et al. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. Sep 16 1999;341(12):871-8. [Medline].
- Marriot HJ, Conover MB. Advanced Concepts in Arrhythmias. 3rd ed. Philadelphia, Pa: Mosby Inc; 1998.
- Part 7.3: Management of Symptomatic Bradycardia and Tachycardia. Circulation. 2005/11;112:IV-67-IV-77. [Full Text].
- Stevenson WG. Catheter ablation of monomorphic ventricular tachycardia. Curr Opin Cardiol. Jan 2005;20(1):42-7. [Medline].
- Stewart RB, Bardy GH, Greene HL. Wide complex tachycardia: misdiagnosis and outcome after emergent therapy. Ann Intern Med. Jun 1986;104(6):766-71. [Medline].
- Testa A, Ojetti V, Migneco A, et al. Use of amiodarone in emergency. Eur Rev Med Pharmacol Sci. May-Jun 2005;9(3):183-90. [Medline].
- Walter PF. Cardiac arrhythmias: Their identification and management. In: Emory University Comprehensive Board Review in Internal Medicine. Vol 1. 1997.
- Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. Sep 5 2006;114(10):e385-484. [Medline].
Keywords
ventricular tachycardia, VT, tachydysrhythmia, ventricular ectopic focus, fusion beats, atrioventricular dissociation, AV dissociation, wide QRS complexes, ventricular fibrillation, VF, paroxysmal supraventricular tachycardia, PSVT, torsade de pointes, accelerated idioventricular rhythm, congestive heart failure, pulmonary edema, jugular venous distention,hypotension, CAD, structural heart disease, hypokalemia, hypocalcemia, hypomagnesia, methamphetamine, cocaine, genetic arrhythmia syndrome, cardiac channelopathy
Contributor Information and Disclosures
Author
Ian S deSouza, MD, Assistant Professor, Department of Emergency Medicine, Kings County Hospital/SUNY Downstate Medical Centers
Ian S deSouza, MD is a member of the following medical societies: American Academy of Emergency Medicine
Disclosure: Nothing to disclose
Coauthor
Che’ Damon Ward, MD, Staff Physician, Department of Emergency Medicine, State University of New York Health Science Center at Brooklyn
Che’ Damon Ward, MD is a member of the following medical societies: American Academy of Emergency Medicine and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose
Medical Editor
Steven A Conrad, MD, PhD, Chief, Department of Emergency Medicine; Chief, Multidisciplinary Critical Care Service, Professor, Department of Emergency and Internal Medicine, Louisiana State University Health Sciences Center
Steven A Conrad, MD, PhD is a member of the following medical societies: American College of Chest Physicians, American College of Critical Care Medicine, American College of Emergency Physicians, American College of Physicians, International Society for Heart and Lung Transplantation, Louisiana State Medical Society, Shock Society, Society for Academic Emergency Medicine, and Society of Critical Care Medicine
Disclosure: Nothing to disclose
Pharmacy Editor
Francisco Talavera, PharmD, PhD, Senior Pharmacy Editor, eMedicine
Disclosure: Nothing to disclose
Managing Editor
Gary Setnik, MD, Chair, Department of Emergency Medicine, Mount Auburn Hospital; Assistant Professor, Division of Emergency Medicine, Harvard Medical School
Gary Setnik, MD is a member of the following medical societies: American College of Emergency Physicians and National Association of EMS Physicians
Disclosure: Nothing to disclose
CME Editor
John D Halamka, MD, MS, Associate Professor of Medicine, Harvard Medical School, Beth Israel Deaconess Medical Center; Chief Information Officer, CareGroup Healthcare System and Harvard Medical School; Attending Physician, Division of Emergency Medicine, Beth Israel Deaconess Medical Center
John D Halamka, MD, MS is a member of the following medical societies: American College of Emergency Physicians, American Medical Informatics Association, Phi Beta Kappa, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose
Chief Editor
David FM Brown, MD, Assistant Professor, Department of Medicine, Division of Emergency Medicine, Harvard Medical School; Associate-Chief, Attending Physician, Department of Emergency Medicine, Massachusetts General Hospital
David FM Brown, MD is a member of the following medical societies: American College of Emergency Physicians and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose
Acknowledgments
The authors and editors of eMedicine gratefully acknowledge the contributions of previous authors, William Ernoehazy, Jr, MD, and Keith Marill, MD, and previous editor, Charles V Pollack, Jr, MD, to the development and writing of this article.
© 1994-2009 by Medscape.
All Rights Reserved
(http://www.medscape.com/public/copyright)
Tags: Rianimazione




One Response to “eMedicine: Ventricular Tachycardia”
Trackbacks/Pingbacks
Leave a Reply
You must be logged in to post a comment.