Thomas M. Kessler, MD Clare J. Fowler, FRCP
Nat Clin Pract Urol 5(12):, 2008. © 2008 Nature Publishing Group
Urinary retention without an identifiable urological cause presents a diagnostic and therapeutic challenge. Patients with nonobstructive chronic urinary retention usually have to rely on intermittent self-catheterization or indwelling suprapubic or transurethral catheters, which significantly affect quality of life. For some patients, however, sacral neuromodulation (SNM) offers an effective therapeutic alternative, and women with primary disorder of urethral sphincter relaxation (Fowler’s syndrome) seem to respond particularly well to this treatment. Although the mechanism of action of SNM is not well understood and requires further investigation, it seems to involve afferent mediation of spinal cord reflexes and brain networks. The evolution of SNM devices and improvements in surgical and testing techniques, especially the introduction of the two-stage tined lead procedure, have considerably reduced the failure, adverse event and surgical revision rates associated with SNM, ensuring that this modality is an effective minimally invasive treatment for urinary retention.
Introduction
Sacral neuromodulation (SNM) was developed in the early 1980s by Tanagho and Schmidt,[1] and has become a well-established treatment modality for patients with refractory lower urinary tract dysfunction, such as nonobstructive chronic urinary retention, urgency–frequency syndrome and urgency incontinence.[1-7] SNM uses a continuous or cycling mode of electrical pulses to activate or inhibit neural reflexes associated with lower urinary tract function via stimulation of the sacral nerves, which innervate the lower urinary tract and pelvic floor. This article reviews the technological developments, outcomes, and mechanism of action of SNM therapy, focusing on its use in patients with urinary retention.
Causes of Urinary Retention
Urinary retention can be caused by detrusor underactivity, bladder outlet obstruction (BOO), or a combination of both (Box 1). For identification of the underlying cause, pressure flow studies are necessary.[8] Neurogenic and myogenic factors are involved in the pathogenesis of detrusor underactivity, which can also be caused by pharmacotherapy (i.e. drugs with antimuscarinic activity or opiates) or by detrusor inhibition resulting from a primary disorder of urethral sphincter relaxation (Fowler’s syndrome).[9] BOO is a urodynamic diagnosis, and is the generic term for voiding obstruction characterized by increased detrusor pressure and reduced urinary flow rate.[10] Urinary retention caused by BOO can be classified as having either a mechanical–anatomical or a functional (nonmechanical, nonanatomical) basis. Mechanical–anatomical causes include tumors (e.g. prostate, bladder, gynecological or intestinal), bladder neck stenosis or urethral stricture, urethral diverticulum, bladder or urethral calculi, urogenital prolapse, malformation, and previous surgical interventions (especially anti-incontinence procedures). The pathophysiology of functional etiologies is poorly understood, but might involve detrusor bladder neck dyssynergia, detrusor external sphincter dyssynergia, dysfunctional voiding and nonrelaxing urethral sphincter obstruction.
Urinary retention caused by mechanical–anatomical BOO is usually treated successfully with medication or surgery, whereas the management of urinary retention resulting from detrusor underactivity or functional BOO remains a challenge. Once a urological cause has been excluded, by urological examination, urodynamic studies, urethrocystoscopy, and radiological investigation, a neurological basis should be considered (e.g. spinal cord disease, sacral radiculopathy, small fiber neuropathy) and excluded, by neurological examination, MRI, and electroneuromyography. Although various neurological disorders (including spinal cord disease or trauma, multiple sclerosis, small fiber neuropathy and cauda equina syndrome) can cause urinary retention or incomplete emptying, other symptoms or signs usually accompany the urinary complaints if the problem is neurogenic. The greatest diagnostic challenge occurs when no urological or neurological abnormalities are apparent and the results of all investigations are essentially normal. In women, Fowler’s syndrome should then be considered. Women with this disorder show abnormal needle electromyographic signals containing both complex repetitive discharges (sounding like a helicopter or machinegun fire; Supplementary Audio 1 online) and decelerating bursts (reminiscent of whale song; Supplementary Audio 2 online), and half of them have polycystic ovaries on ultrasonography.[9]
Patients with urinary retention caused by detrusor underactivity or functional BOO usually have to rely on intermittent self-catheterization or indwelling suprapubic or transurethral catheters, which significantly affects quality of life. For some of these patients, SNM offers an effective therapeutic alternative, and women with Fowler’s syndrome seem to respond particularly well to this treatment.[11]
Evolution of SNM Technology and Surgical Technique
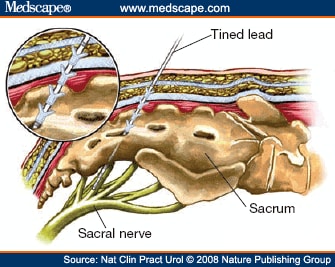
SNM technology and surgical technique have undergone major developments in the past decade.[12] Traditionally, after surgical placement of a temporary lead (unipolar wire electrode) in the sacral foramen S3, a test stimulation for 4–7 days, known as ‘percutaneous’ or ‘peripheral’ nerve evaluation (PNE), was performed. The lead was taped to the exterior skin surface but did not have any fixation at deeper levels, putting the patient at risk for lead migration. Patients with a successful test phase (>50% symptom improvement assessed by bladder diary) underwent subsequent simultaneous implantation of both a quadripolar definitive lead and an implantable pulse generator (IPG) in an open, one-stage procedure. A medial incision over the sacrum was carried down to the level of the sacral periosteum, to which a plastic anchor was affixed to hold the definitive lead in place. The cable connections to the IPG were then tunneled to an IPG pocket on the anterior abdominal wall.
With this traditional, one-stage procedure, the patient’s positive response to PNE was occasionally not reproduced after definitive lead placement because of inevitable slight differences in position between the temporary (unipolar electrode) and definitive (quadripolar electrode) leads. Thus, to minimize the risk of this technical failure, a two-stage implantation technique was devised by Janknegt and colleagues,[13] who used the definitive lead for both test and permanent stimulation. Other attempts to improve outcomes have included direct bilateral attachment of leads to the sacral nerves via a sacral laminectomy,[14] and tailored laminectomy for bilateral lead implantation.[15] The recent trend, however, has been in favor of less-invasive procedures. Spinelli and colleagues[16,17] modified the two-stage technique to place the lead within the sacral foramen next to the sacral nerve root using a minimally invasive, percutaneous approach, without the need to incise overlying muscle and fascia. This procedure was initially performed using anchoring to the lumbodorsal fascia.[16] With the introduction of the tined lead (see Figure 1), no additional anchoring has been needed and SNM lead placement has become a truly minimally invasive procedure that can be performed under local anesthesia (Figure 1 and 2).[17]
Placement of the IPG on the anterior abdominal wall is often associated with discomfort at the IPG site, and prolongs the surgical procedure. Nowadays, therefore, the IPG is implanted in most patients in the subcutaneous tissue of the buttock region (Figure 3). Such placement has been found to simplify the surgical procedure and to reduce the rate of reoperation for pain;[18] however, no randomized study has compared anterior abdominal wall versus buttock region placement of the IPG, so the true effect on the reoperation rate remains unclear. The most widely used IPG device has been the InterStim® Model 3023 (Medtronic Inc., Minneapolis, MN; CE mark November 1995). A smaller IPG device (InterStim® II Model 3058; CE mark April 2006), which does not need an extension cable for connection to the tined lead, is now available, allowing for a smaller incision and IPG pocket, which might result in less discomfort and, therefore, higher patient acceptance, especially among slim patients.[19] The estimated battery lifetime of the new IPG device is, however, considerably shorter than that of the older one (InterStim® II Model 3058, 2.5–5.0 years; InterStim® Model 3023, 5–8 years). The new IPG device is also more expensive than the older one (list prices: InterStim® II Model 3058, €7,550 [US$10,340]; InterStim® Model 3023, €6,100 [$8,350] plus €660 [$905] for a required 10 cm extension cable); whether SNM therapy is reimbursable varies widely between (and sometimes within) different countries.
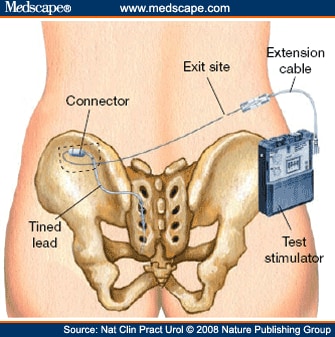
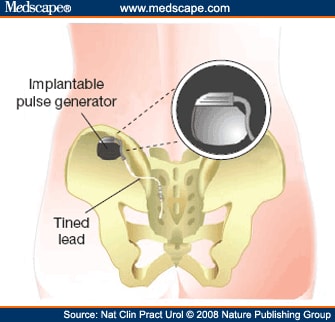 |
Figure 3. (click image to zoom) Permanent implantation. Patients with successful results of the test phase undergo implantation of the implantable pulse generator. The extension cable is removed and the permanent stimulator is implanted in the buttock region and connected to the tined lead. Reprinted with the permission of Medtronic, Inc. © 2006.
|
Outcomes of SNM for Urinary Retention
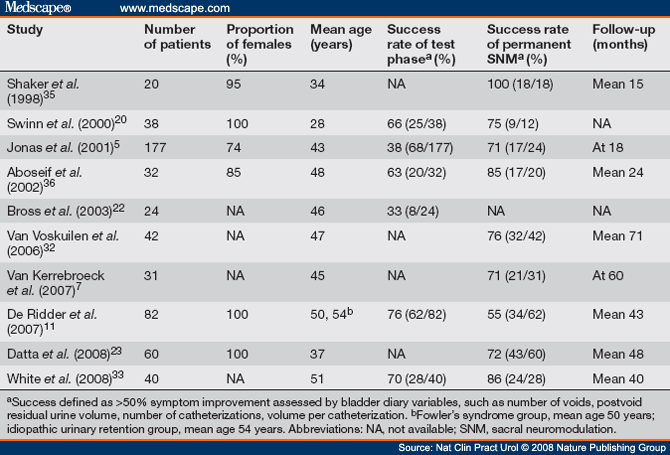
Most studies reporting on the use of SNM for urinary retention are case series (Table 1), but one prospective, multicenter, randomized controlled trial has been performed, which provides the most convincing evidence for the efficacy of SNM in this group of patients.[5] Across these studies, the mean duration of follow-up ranges from 15 to 71 months. We should note that, in the published literature, the cause of urinary retention was often not specified, and most patients treated with SNM for urinary retention were women. Moreover, no study has investigated the differences in outcome and adverse events between sexes.
Efficacy
Improvement of more than 50% in bladder diary variables (e.g. number of voids, postvoid residual urine volume, number of catheterizations and volume per catheterization) is usually considered a positive test stimulation result, and an indication for implantation of the IPG; as a result, efficacy outcomes can generally be compared between studies. The success rate of traditional PNE in patients with nonobstructive, chronic urinary retention has been reported in the range 33–66%. [5,20-23] This rate has been markedly improved to almost 79% by a recently described technical modification, involving subcutaneous tunneling of the temporary lead, in a mixed group of patients.[24] Some patients with successful PNE, however, do not continue to have positive clinical effects after IPG implantation,[5] whereas some patients with unsuccessful PNE still respond well to SNM.[13,23,25] Efforts should be made, therefore, to further optimize patient selection through identification of predictive factors and improvement of the testing technique. Other than test results, generally accepted predictive factors for patient response are still lacking, although neurophysiology[26] and pretreatment ability to void[27] have been suggested. In a randomized trial that included only patients with urgency incontinence, patients allocated to receive tined lead testing were more likely to progress to IPG implantation than were those who received traditional PNE.[28] The advantages of definitive lead testing versus traditional PNE are that the definitive lead is less prone to migration, it allows for a longer test phase and, in the case of positive test results, the lead remains in the same effective position. Thus, the eligibility for IPG implantation significantly increased from 50% after traditional PNE (4–7 days) to 80% after a prolonged (minimum of 14 days) test phase using definitive leads;[29] prolonged testing seemed not to increase the rate of complications.[30] Moreover, a randomized trial has shown that the two-stage implantation technique has higher short-term and long-term success rates than the standard one-stage procedure following a positive PNE.[31]
In a multicenter trial that included 68 patients with nonobstructive, chronic urinary retention qualifying for IPG implantation (≥50% reduction in catheter volume per catheterization during PNE), 37 individuals were randomly assigned to undergo immediate IPG implantation and 31 were assigned to a control group with delayed IPG implantation after 6 months.[5] IPGimplanted patients had a significant reduction in the catheter volume per catheterization and the number of catheterizations, as well as a significant increase in the volume voided and the number of voids per day. At 6 months, improvement was seen in 24 of 29 (83%) implanted patients (20 of whom had stopped using catheters and 4 who had a reduction of ≥50% in catheter volume per catheterization) compared with only 2 of 22 (9%) control patients. The beneficial effects of SNM were sustained at 18 months after implantation, and temporary inactivation of SNM resulted in a significant increase in postvoid residual urine volume.
Long-term results of SNM have been reported by several groups. Of 60 patients with Fowler’s syndrome, SNM was successful in 43 (72%), with a follow-up duration of up to 10 years (mean follow-up 48 months).[23] This result is in line with the reported success rates of 76% (32 of 42 patients) and 71% (22 of 31 patients) for patients with nonobstructive chronic urinary retention with follow-up at a mean of 71 months in a retrospective, single-center study[32] and at 5 years in a prospective, multicenter trial,[7] respectively. In addition, an even higher success rate of 86% (24 of 28 patients) for SNM in patients with nonobstructive urinary retention was reported in another retrospective single center study with a mean follow-up duration of 40 months.[33] Thus, considering the different lower urinary tract disorders (i.e. chronic urinary retention, urgency– frequency syndrome, and urgency incontinence), the best long-term results for SNM are achieved in patients with chronic urinary retention;[7,34] the presence of Fowler’s syndrome seems to be a predictive factor for success,[11] although the reason for this is not known.
Notably, SNM not only restores voiding function, but also improves quality of life[35-37] and reduces voiding-related health-care costs.[38] In a mixed group of patients who were not differentiated according to the type of lower urinary tract dysfunction, IPG implantation resulted in a 92% first-year reduction in costs associated with outpatient doctor visits and diagnostic and therapeutic procedures, along with a 30% reduction in drug expenditure.[38]
Safety
Although SNM is a minimally invasive treatment, it does involve surgery and, therefore, is associated with some risks. Complications include lead or IPG migration, infection, pain at the lead or IPG site, leg pain, nerve irritation, bowel dysfunction, technical problems, and loss of effect. [3,6,7,23,39-42] The adverse event rate during the test phase is about 18% for PNE,[3] and 6% for tined lead testing.[30] During and following IPG implantation, adverse event rates as high as 67%[7] have been reported for the one-stage procedure (with a surgical revision rate as high as 73%);[23] for the two-stage approach, adverse events rates have been reported to be as high as 23%[43] (with a surgical revision rate as high as 33%).[23]
Importantly, despite the high incidence of reported adverse events, no account exists in the literature of any life-threatening complication or death (i.e. grade 4 or 5 complications according to the classification of Dindo and colleagues)[44] attributable to SNM for lower urinary tract dysfunction. In early studies, revision surgery was performed in most patients who experienced a loss of therapeutic effect, but this procedure is often not effective.[45] Adverse event and surgical revision rates have decreased considerably during recent years, reflecting the increasing expertise gained by physicians and the improvements made in both implantation techniques and devices. For example, the rate of lead-associated adverse events was markedly lower for tined than for nontined leads (28% vs 73%).[46] In addition, a significant difference in surgical revision rates was found in patients implanted before versus after 1995 (revision/implantation ratio 1.56 vs 0.49).[32]
Troubleshooting
If adverse events or loss of efficacy occur following initially successful SNM, explantation of the lead or IPG can be considered. Surgery, however, is not necessary in all patients. Problems attributable to lead migration can often be resolved by reprogramming, additional fixation of the lead, or contralateral insertion of a new lead.[47] In the case of adverse stimulation, it might be sufficient to change the stimulation parameters, such as electrode configuration, pulse width, amplitude, mode or polarity. Despite the tines, lead migration can occur after tined lead placement; [41,47-49] however, revision surgery is not justified on the basis of radiological findings alone. Provided a part of the lead remains in the foramen and a positive clinical effect of SNM is maintained, no treatment is needed. Thus, reprogramming and impedance checking before radiological investigation seems reasonable.
In addition, the IPG needs to be replaced when the battery expires. Although this represents a minor surgical procedure and can be performed under local anesthesia, patients must be informed about this need before embarking on SNM.
Bilateral SNM
The role of bilateral SNM is still unclear, and requires further evaluation. Unilateral SNM is sufficient in most patients, perhaps because of the prominent dorsal commissure in the sacral cord, which presumably has a powerful integrating function at a segmental level. In a randomized crossover trial in 12 patients with urgency incontinence and 13 with urinary retention,[50] no significant difference in improvement was found between unilateral and bilateral testing using traditional, temporary leads. Two patients with urinary retention were, however, able to void only during bilateral stimulation. Thus, if unilateral SNM fails, a bilateral test should be considered. Another promising treatment option after failed SNM is bilateral caudal epidural neuromodulation, whereby bilateral tined leads are placed into the caudal epidural space in a retrograde approach. The basic theory behind this procedure is not only to bilaterally stimulate the sacral roots at the S3 level, but also at the S2 and S4 levels, as these are also involved in lower urinary tract and pelvic floor function. This approach recruits more neural pathways and might increase therapeutic efficacy. Indeed, bilateral caudal epidural neuromodulation was found to restore voiding in 5 (63%) of 8 patients in whom prior unilateral (6 patients) or bilateral (2 patients) SNM failed, and no significant adverse events were reported.[51] These results, however, need to be reproduced by other investigators and in larger series before bilateral caudal epidural neuromodulation can be considered a valuable treatment option for patients with urinary retention.
Mechanism of Action of SNM in Urinary Retention
Neuromodulation is a process whereby an electrical stimulus or pharmaceutical agent modulates the pre-existing activity in a neural pathway. Although the mechanism of action of SNM is not well understood, it seems to involve modulation of spinal cord reflexes and brain networks by peripheral afferents, rather than direct stimulation of the motor response of the detrusor or urethral sphincter. Even the anal sphincter contraction (the bellows response) that is used to monitor SNM lead placement is not a direct stimulation effect, but rather is an afferent- mediated response.[52,53] Moreover, the amplitudes of the electrical current applied during permanent SNM are considerably lower than the activation threshold of somatic muscle.[54] For this reason, ‘sacral neuromodulation’ (SNM) rather than ‘sacral nerve stimulation’ (SNS) is now the preferred term for this therapy.
Studies in animals have suggested various mechanisms of action of SNM. In a rat model of chemical cystitis, SNM reduced the frequency of bladder contractions, and neuromodulatory effects could be attenuated or abolished by superfusion of the spinal cord with an antagonist of non-N-methyl-d-aspartate (non-NMDA) receptors (i.e. receptors for glutamate, the most common neurotransmitter released in the spinal cord).[55,56] Thus, changes in spinal cord signal processing might be important in SNM. In addition, nitric oxide metabolites might have a role, as SNM increased the expression of urothelial and endothelial inducible nitric oxide synthase (iNOS) and urothelial neuronal nitric oxide synthase (nNOS) in the rat bladder.[57] In experimental models of spinal cord injury, SNM seems to work by blocking afferent C-fiber activity. In spinally transected rats, SNM has been shown to reduce the upregulated expression of c-fos, substance P, neurokinin A, calcitonin gene-related peptide and vanilloid receptor 1 in the spinal cord, and inhibit detrusor activity.[58-60] SNM did not, however, inhibit afferent C-fiber activity in rats with hydrochloric-acid-induced cystitis, although micturition frequency was reduced.[61]
In humans, possible mechanisms of action of SNM have been assessed by urodynamic studies, neurophysiological investigations, functional brain imaging, and measurement of biomarkers. One long-standing question is that of how SNM can show efficacy in both urinary retention and overactive bladder syndrome. The explanation seems to lie in the different underlying pathophysiology of these disorders. In patients with detrusor overactivity, SNM is thought to inhibit detrusor activity without affecting urethral resistance or the strength of detrusor contractions during voiding.[62] The observation that early, bilateral SNM initiated during spinal shock could prevent the development of detrusor overactivity in complete spinal cord injury[63] might indicate modulation at the level of the spinal cord itself. In a PET study of patients with urgency incontinence, differences between the effects of acute and chronic SNM were noted in the associative sensory cortex, premotor cortex, and the cerebellum.[64] Thus, the authors suggested that acute SNM predominantly involves areas associated with sensorimotor learning, which might become progressively less active during the course of chronic SNM. By contrast, in urinary retention, SNM has been postulated to inhibit inappropriate activation of the ‘guarding reflex’ (i.e. the spinally mediated reflex whereby the urethral sphincter contracts to prevent urinary incontinence on sudden increase in intravesical pressure), thus facilitating voiding by interrupting the excitatory outflow to the urethral sphincter.[5,65] In a study of 30 women with Fowler’s syndrome, however, elevated maximum urethral closure pressure did not change significantly, and electromyographic abnormality persisted during SNM; the return of voiding ability seemed to be attributable to a slight increase in detrusor contractility.[66] At least in women with Fowler’s syndrome, SNM seems not to restore voiding by a direct relaxant effect on the urethral sphincter, but instead by an increase in detrusor contractility that is sufficient to overcome the still overactive urethral sphincter. Thus, SNM might work by releasing the detrusor from presumed inhibition emanating from urethral sphincter afferent activity. This suggestion is consistent with the results of a PET study investigating healthy controls and women with Fowler’s syndrome.[67] Bladder filling increased the activity in the midbrain and limbic cortical regions in healthy controls, but women with Fowler’s syndrome lacked brainstem activation and showed enhanced limbic cortical activity when the bladder was full. SNM restored a normal pattern of midbrain activity and decreased cortical activity, suggesting that the effect of SNM in women with Fowler’s syndrome depends on afferent input to midbrain centers involved in initiating micturition.
Another hypothesis is that the sudden cessation of continuous SNM induces detrusor contraction and spontaneous voiding via a ‘rebound phenomenon’ in patients with urinary retention.[68,69] By analogy to pharmacological experiments in which the removal of a drug that has induced desensitization of receptors results in a transient increase of the receptor effect, the SNM rebound phenomenon might involve an increase in previously inhibited efferent detrusor activity after the removal of the inhibitory stimulus. Fundamental differences exist, however, between the animal model studied[69] (cat) and humans; furthermore, SNM is successfully used for treating urinary retention at most centers without switching off the IPG before voiding.
The importance of afferent pathways in SNM is further supported by several neurophysiological studies. In one study, SNM reduced the sensory threshold of the bladder, but not of the urethra, suggesting the involvement of the afferent nervous system through the pelvic plexus.[70] Following SNM, a cortical potential complex was demonstrated in the electroencephalograms of all patients, irrespective of whether a patient was aware of the neuromodulator being switched on and off–a finding compatible with SNM involving the sensory cortex areas.[71] SNM was associated with a small but significant decrease in pudendal somatosensory evoked potential latency.[26] Moreover, the sympathetic nervous system might have a role, as indicated by studies of low-frequency pudendal nerve stimulation in cats with chronic spinal cord injury[72] and the observation of inhibited colonic activity and enhanced internal anal sphincter activity during SNM in cats.[73] Whether the sympathetic nervous system is also involved in SNM for urinary retention needs further evaluation.
Biomarkers might be useful in future studies investigating the mechanism of action of SNM. A single study of SNM in patients with interstitial cystitis has shown that improvement of symptoms was associated with normalization of urinary heparin-binding epidermal growth factor-like growth factor (HB-EGF) and antiproliferative factor activity.[74] In addition, patients with fecal incontinence have shown normalization of previously elevated rectal mucosal substance P levels following successful SNM.[75]
Conclusions
In selected patients with urinary retention caused by detrusor underactivity or functional BOO, SNM offers an important therapeutic alternative to intermittent self-catheterization or indwelling suprapubic or transurethral catheters. Women with Fowler’s syndrome seem to respond best to this treatment. SNM not only restores voiding function, but also improves quality of life and reduces voiding-related health-care costs. The evolution of SNM devices and improvements in surgical and testing techniques, especially the introduction of the two-stage tined lead procedure, have considerably reduced the failure, adverse event and surgical revision rates associated with SNM, and have made this modality an effective, minimally invasive treatment for urinary retention. If unilateral SNM fails, a bilateral test should be considered. Bilateral caudal epidural neuromodulation might become a valuable treatment option in the case of failed SNM. Although the mechanism of action of SNM is not well understood and requires further investigation, it seems to involve afferent mediation of spinal cord reflexes and brain networks.
Supplementary information in the form of audio files is available on the Nature Clinical Practice Urology website.
Sidebar: Key Points
- In selected patients with chronic urinary retention, sacral neuromodulation (SNM) offers an important therapeutic alternative to intermittent self-catheterization or indwelling suprapubic or transurethral catheters, which significantly affect quality of life
- SNM might be an effective treatment for restoring voiding function, thereby improving quality of life and reducing voiding-related health-care costs
- Technical improvements in devices and changes in surgical and testing techniques have considerably reduced failure, adverse event and surgical revision rates, and have made SNM an effective, minimally invasive treatment
- Although the mechanism of action of SNM is not well understood, it seems to involve modulation of spinal cord reflexes and brain networks by peripheral afferents
Funding Information
TM Kessler is supported by a grant from the Swiss National Science Foundation.
Acknowledgments
The work for this Review was undertaken at University College London Hospitals–University College London, which have received a proportion of funding from the UK Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Reprint Address
Department of Uro-Neurology, The National Hospital for Neurology and Neurosurgery, University College London Hospitals NHS Foundation Trust, Queen Square, London WC1N 3BG, UK; Email: tkessler@gmx.ch
Tables
Table 1. Main Studies of Sacral Neuromodulation for Urinary Retention
Box 1. Causes of Urinary Retention
References
- Tanagho EA and Schmidt RA (1982) Bladder pacemaker: scientific basis and clinical future. Urology 20: 614–619
- Hassouna MM et al. (2000) Sacral neuromodulation in the treatment of urgency-frequency symptoms: a multicenter study on efficacy and safety. J Urol 163: 1849–1854
- Siegel SW et al. (2000) Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgencyfrequency, and retention. Urology 56: 87–91
- Bosch JL and Groen J (2000) Sacral nerve neuromodulation in the treatment of patients with refractory motor urge incontinence: long-term results of a prospective longitudinal study. J Urol 163: 1219–1222
- Jonas U et al. (2001) Efficacy of sacral nerve stimulation for urinary retention: results 18 months after implantation. J Urol 165: 15–19
- Kessler TM et al. (2007) Sacral neuromodulation for refractory lower urinary tract dysfunction: results of a nationwide registry in Switzerland. Eur Urol 51: 1357–1363
- van Kerrebroeck PE et al. (2007) Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178: 2029–2034
- Takeda M et al. (2003) Diagnosis and treatment of voiding symptoms. Urology 62: 11–19
- Fowler CJ et al. (1988) Abnormal electromyographic activity of the urethral sphincter, voiding dysfunction, and polycystic ovaries: a new syndrome? BMJ 297: 1436–1438
- Abrams P et al. (2002) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21: 167–178
- De Ridder D et al. (2007) The presence of Fowler’s syndrome predicts successful long-term outcome of sacral nerve stimulation in women with urinary retention. Eur Urol 51: 229–233; discussion 233–234
- Spinelli M and Sievert KD (2008) Latest technologic and surgical developments in using InterStim™ therapy for sacral neuromodulation: impact on treatment success and safety. Eur Urol [doi:10.1016/ j.eururo.2008.01.076]
- Janknegt RA et al. (1997) Improving neuromodulation technique for refractory voiding dysfunctions: two-stage implant. Urology 49: 358–362
- Hohenfellner M et al. (1998) Bilateral chronic sacral neuromodulation for treatment of lower urinary tract dysfunction. J Urol 160: 821–824
- Braun PM et al. (1999) Tailored laminectomy: a new technique for neuromodulator implantation. J Urol 162: 1607–1609
- Spinelli M et al. (2003) New percutaneous technique of sacral nerve stimulation has high initial success rate: preliminary results. Eur Urol 43: 70–74
- Spinelli M et al. (2003) New sacral neuromodulation lead for percutaneous implantation using local anesthesia: description and first experience. J Urol 170: 1905–1907
- Scheepens WA et al. (2001) Buttock placement of the implantable pulse generator: a new implantation technique for sacral neuromodulation–a multicenter study. Eur Urol 40: 434–438
- Sievert KD et al. (2007) Permanente sakrale Neuromodulation mittels InterStim®: Ergebnisse einer Anwenderbefragung zu aktuellen technischen Entwicklungen. J Urol Urogynäkol 4: 14–16
- Swinn MJ et al. (2000) Sacral neuromodulation for women with Fowler’s syndrome. Eur Urol 38: 439–443
- Aboseif S et al. (2002) Sacral neuromodulation as an effective treatment for refractory pelvic floor dysfunction. Urology 60: 52–56
- Bross S et al. (2003) The role of the carbachol test and concomitant diseases in patients with nonobstructive urinary retention undergoing sacral neuromodulation. World J Urol 20: 346–349
- Datta SN et al. (2008) Sacral neurostimulation for urinary retention: 10-year experience from one UK centre. BJU Int 101: 192–196
- Sievert KD et al. (2007) Subcutaneous tunneling of the temporary testing electrode significantly improves the success rate of subchronic sacral nerve modulation (SNM). World J Urol 25: 607–612
- Scheepens WA et al. (2002) Long-term efficacy and safety results of the two-stage implantation technique in sacral neuromodulation. BJU Int 90: 840–845
- Malaguti S et al. (2003) Neurophysiological evidence may predict the outcome of sacral neuromodulation. J Urol 170: 2323–2326
- Goh M and Diokno AC (2007) Sacral neuromodulation for nonobstructive urinary retention–is success predictable? J Urol 178: 197–199
- Borawski KM et al. (2007) Predicting implantation with a neuromodulator using two different test stimulation techniques: a prospective randomized study in urge incontinent women. Neurourol Urodyn 26: 14–18
- Kessler TM et al. (2005) Prolonged sacral neuromodulation testing using permanent leads: a more reliable patient selection method? Eur Urol 47: 660–665
- Kessler TM et al. (2008) Safety of prolonged sacral neuromodulation tined lead testing. Curr Med Res Opin 24: 343–347
- Everaert K et al. (2004) A prospective randomized trial comparing the 1-stage with the 2-stage implantation of a pulse generator in patients with pelvic floor dysfunction selected for sacral nerve stimulation. Eur Urol 45: 649–654
- van Voskuilen AC et al. (2006) Long term results of neuromodulation by sacral nerve stimulation for lower urinary tract symptoms: a retrospective single center study. Eur Urol 49: 366–372
- White WM et al. (2008) Sacral nerve stimulation for treatment of refractory urinary retention: long-term efficacy and durability. Urology 71: 71–74
- Elhilali MM et al. (2005) Sacral neuromodulation: longterm experience of one center. Urology 65: 1114–1117
- Shaker HS and Hassouna M (1998) Sacral root neuromodulation in idiopathic nonobstructive chronic urinary retention. J Urol 159: 1476–1478
- Aboseif S et al. (2002) Sacral neuromodulation in functional urinary retention: an effective way to restore voiding. BJU Int 90: 662–665
- Das AK et al. (2004) Improvement in depression and health-related quality of life after sacral nerve stimulation therapy for treatment of voiding dysfunction. Urology 64: 62–68
- Aboseif SR et al. (2007) Sacral neuromodulation: cost considerations and clinical benefits. Urology 70: 1069–1073
- Swinn MJ et al. (1999) Leg pain after sacral neuromodulation: anatomical considerations. BJU Int 84: 1113–1115
- Spinelli M et al. (2001) Chronic sacral neuromodulation in patients with lower urinary tract symptoms: results from a national register. J Urol 166: 541–545
- Hijaz A et al. (2006) Complications and troubleshooting of two-stage sacral neuromodulation therapy: a single-institution experience. Urology 68: 533–637
- Brazzelli M et al. (2006) Efficacy and safety of sacral nerve stimulation for urinary urge incontinence: a systematic review. J Urol 175: 835–841
- Van Voskuilen AC et al. (2007) Medium-term experience of sacral neuromodulation by tined lead implantation. BJU Int 99: 107–110
- Dindo D et al. (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240: 205–213
- Everaert K et al. (2000) Patient satisfaction and complications following sacral nerve stimulation for urinary retention, urge incontinence and perineal pain: a multicenter evaluation. Int Urogynecol J Pelvic Floor Dysfunct 11: 231–235
- Sutherland SE et al. (2007) Sacral nerve stimulation for voiding dysfunction: one institution’s 11-year experience. Neurourol Urodyn 26: 19–28
- Deng DY et al. (2006) Failure of sacral nerve stimulation due to migration of tined lead. J Urol 175: 2182–2185
- Kessler TM et al. (2005) Bilateral migration of sacral neuromodulation tined leads in a thin patient. J Urol 173: 153–154
- Spinelli M et al. (2005) New tined lead electrode in sacral neuromodulation: experience from a multicentre European study. World J Urol 23: 225–229
- Scheepens WA et al. (2002) Unilateral versus bilateral sacral neuromodulation in patients with chronic voiding dysfunction. J Urol 168: 2046–2050
- Maher MG et al. (2007) Bilateral caudal epidural neuromodulation for refractory urinary retention: a salvage procedure. J Urol 177: 2237–2240
- Fowler CJ et al. (2000) Studies of the latency of pelvic floor contraction during peripheral nerve evaluation show that the muscle response is reflexly mediated. J Urol 163: 881–883
- Schurch B et al. (2003) Electrophysiological recordings during the peripheral nerve evaluation (PNE) test in complete spinal cord injury patients. World J Urol 20: 319–322
- van der Pal F et al. (2006) Current opinion on the working mechanisms of neuromodulation in the treatment of lower urinary tract dysfunction. Curr Opin Urol 16: 261–267
- Riazimand SH and Mense S et al. (2004) A rat model for studying effects of sacral neuromodulation on the contractile activity of a chronically inflamed bladder. BJU Int 94: 158–163
- Riazimand SH and Mense S (2005) Interaction between neurotransmitter antagonists and effects of sacral neuromodulation in rats with chronically hyperactive bladder. BJU Int 96: 900–908
- Minardi D et al. (2008) Activity and expression of nitric oxide synthase in rat bladder after sacral neuromodulation. Int J Immunopathol Pharmacol 21: 129–135
- Wang Y and Hassouna MM (2000) Neuromodulation reduces c-fos gene expression in spinalized rats: a double-blind randomized study. J Urol 163: 1966–1970
- Shaker H et al. (2000) Role of C-afferent fibres in the mechanism of action of sacral nerve root neuromodulation in chronic spinal cord injury. BJU Int 85: 905–910
- Zhou Y et al. (2002) Change of vanilloid receptor 1 following neuromodulation in rats with spinal cord injury. J Surg Res 107: 140–144
- Wang Y et al. (2000) Neuromodulation reduces urinary frequency in rats with hydrochloric acid-induced cystitis. BJU Int 86: 726–730
- Groen J et al. (2006) Sacral neuromodulation in women with idiopathic detrusor overactivity incontinence: decreased overactivity but unchanged bladder contraction strength and urethral resistance during voiding. J Urol 175: 1005–1009
- Sievert KD et al. (2008) The early implantation of bilaterally sacral nerve modulators to prevent the neurogenic bladder malfunction in paraplegic patients [abstract 574]. Eur Urol Suppl 7: 214
- Blok BF et al. (2006) Different brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulators. BJU Int 98: 1238–1243
- Leng WW and Chancellor MB (2005) How sacral nerve stimulation neuromodulation works. Urol Clin North Am 32: 11–18
- DasGupta R and Fowler CJ (2004) Urodynamic study of women in urinary retention treated with sacral neuromodulation. J Urol 171: 1161–1164
- Dasgupta R et al. (2005) Changes in brain activity following sacral neuromodulation for urinary retention. J Urol 174: 2268–2272
- Schultz-Lampel D et al. (1998) Experimental results on mechanisms of action of electrical neuromodulation in chronic urinary retention. World J Urol 16: 301–304
- Bannowsky A et al. (2003) Sacral neuromodulation in treatment of functional disorders of the lower urinary tract. An overview of basic principles, indications, outcomes [German]. Urologe A 42: 1357–1365
- Wyndaele JJ et al. (2000) Influence of sacral neuromodulation on electrosensation of the lower urinary tract. J Urol 163: 221–224
- Braun PM et al. (2002) Alterations of cortical electrical activity in patients with sacral neuromodulator. Eur Urol 41: 562–566
- Tai C et al. (2006) Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol 197: 225–234
- Vitton V et al. (2008) Colonosphincteric electromyographic responses to sacral root stimulation: evidence for a somatosympathetic reflex. Neurogastroenterol Motil 20: 407–416
- Chai TC et al. (2000) Percutaneous sacral third nerve root neurostimulation improves symptoms and normalizes urinary HB-EGF levels and antiproliferative activity in patients with interstitial cystitis. Urology 55: 643–646
- Gooneratne ML et al. (2008) Normalization of substance P levels in rectal mucosa of patients with faecal incontinence treated successfully by sacral nerve stimulation. Br J Surg 95: 477–83
Authors and Disclosures
As an organization accredited by the ACCME, Medscape, LLC requires everyone who is in a position to control the content of an education activity to disclose all relevant financial relationships with any commercial interest. The ACCME defines “relevant financial relationships” as financial relationships in any amount, occurring within the past 12 months, including financial relationships of a spouse or life partner, that could create a conflict of interest.
Medscape, LLC encourages Authors to identify investigational products or off-label uses of products regulated by the US Food and Drug Administration, at first mention and where appropriate in the content.
Author
Thomas M. Kessler, MD
Clinical and Research Fellow, The National Hospital for Neurology and Neurosurgery, University College London Hospitals NHS Foundation Trust, London, United Kingdom Disclosure: Thomas M. Kessler, MD, has disclosed that he has served as a consultant for Medtronic, Inc.
Clare J. Fowler, FRCP
Consultant; Professor of Uro-Neurology; Head of the Department of Uro-Neurology, The National Hospital for Neurology and Neurosurgery, University College London Hospitals NHS Foundation Trust, London, United Kingdom Disclosure: Clare J. Fowler, FRCP, has disclosed that she has served as a consultant for Medtronic, Inc.
CME Author
Charles P. Vega, MD, FAAFP
Associate Professor; Residency Director, Department of Family Medicine, University of California, Irvine Disclosure: Charles P. Vega, MD, has disclosed that he has served as an advisor or consultant to Novartis, Inc.
Editor
Nayanah Siva
Locum Editor, Nature Clinical Practice Urology
Disclosure: Nayanah Siva has disclosed no relevant financial relationships.






One Response to “Sacral Neuromodulation for Urinary Retention”
Trackbacks/Pingbacks
Leave a Reply
You must be logged in to post a comment.