Paravertebral blocks (PVBs) were first performed in 1905[1, 2] and became a popular technique for the provision of analgesia in the early part of the twentieth century. However, their use declined over the years until a publication by Eason and Wyatt[3] in 1979 began a renaissance. Since then, a considerable number of good quality studies have been published on PVB[4–10] and it is now an established regional anaesthetic technique.
Anatomy of the Thoracic Paravertebral Space
The thoracic paravertebral space begins at T1 and extends caudally to terminate at T12. Although PVBs can be performed in the cervical and lumbar regions, there is no direct communication between adjacent levels in these areas. Most PVBs are therefore performed at the thoracic level.
The thoracic paravertebral space is wedge shaped in all three dimensions. The bodies of the vertebrae, intervertebral discs, and intervertebral foraminae form the medial wall. Anterolaterally, the space is bounded by the parietal pleura and the innermost intercostal membrane. Posteriorly, it is bounded by the transverse processes (TPs) of the thoracic vertebrae, heads of the ribs, and the superior costotransverse ligament.
The thoracic paravertebral space is divided into a posterior subendothoracic and an anterior subserous compartment by the endothoracic fascia, the significance of which is unclear.
The paravertebral space contains spinal nerves, white and grey rami communicantes, the sympathetic chain, intercostal vessels, and fat (Fig. 1).
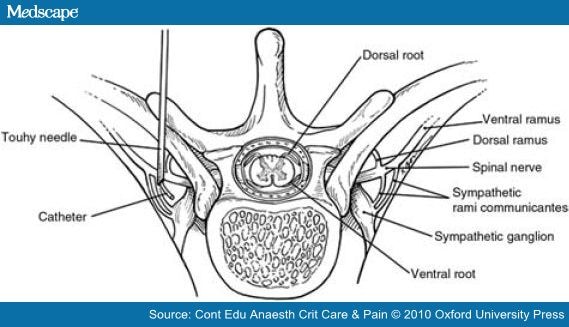
Figure 1. Anatomy of the paravertebral space. Reprinted from Lönnqvist and Richardson.[11] Copyright (1999), with permission from Elsevier.
Indications for PVB
Unilateral Surgical Procedures in the Thoracoabdominal Region
- Breast surgery
- Thoracic surgery
- Cholecystectomy
- Renal surgery
- Appendicectomy
- Inguinal hernia repair
Relief of Acute Pain
- Fractured ribs
- Liver capsule pain (trauma or ruptured cysts)
Relief of Chronic Pain
- Neuropathic chest or abdominal pain (post-surgical or post-herpetic)
- Complex regional pain syndrome
- Refractory angina pectoris
- Relief of cancer pain
Miscellaneous
- Therapeutic control of hyperhydrosis[2]
The use of bilateral PVBs for midline or bilateral surgery has been described but it rarely confers advantages over thoracic epidural anaesthesia.
Contraindications
There are very few absolute contraindications. They include:
- local sepsis (cutaneous or intrathoracic);
- tumours in the paravertebral space at the level of injection;
- allergy to local anaesthetic drugs;
- patient refusal.
Relative contraindications include:
- severe coagulopathy;
- severe respiratory disease (where the patient depends on intercostal muscle function for ventilation);
- ipsilateral diaphragmatic paresis;
- severe spinal deformities (kyphosis or scoliosis). If the anatomy is abnormal, the difficulty and risks increase.
Recommended Technique for PVB
Preparation
Informed consent should be obtained from the patient and i.v. access established. Standard non-invasive monitoring should be applied and the block performed in an area where full resuscitation facilities and a trained assistant are available. Full aseptic precautions should be taken when preparing for the block.
Positioning
If awake, the patient should be seated with the neck and back flexed. If performed under sedation or general anaesthesia, the patient is turned to the lateral position with the operated side uppermost. A bag of saline or a pillow can be placed between the patient and the operating table surface at the level of the intended block, to open up the spaces between adjacent TPs.
Choosing the Level
If only one to four dermatomes need to be blocked, a single level PVB at or below the mid-dermatomal level is usually sufficient (e.g. for simple mastectomy; T3 or T4 is an appropriate level. For open cholecystectomy; T6 or T7 should be selected).
If spread greater than four dermatomes is required, then multiple injections will block the area more reliably (e.g. for mastectomy and axillary dissection, a block from at least T1–T6 will be required. Therefore, blocks should be performed at each level or at T1, T3, and T5).
For blocks in the lumbar region, it is recommended that individual injections be performed at each level with small volumes of local anaesthetic, because spread between adjacent levels is less reliable than in the thoracic region (e.g. for inguinal herniorrhaphy, blocks should be performed at T12, L1, and L2).
Having decided the level and number of blocks to be performed, the skin is marked at the tips of the appropriate spinous processes and at points 25 mm lateral to these. The latter points should correspond with the appropriate TPs. If the block is being performed awake or under sedation, skin infiltration with dilute local anaesthetic will be required. A 21 G needle should then be used to infiltrate down to the TP, taking care not to inject at a depth greater than 35 mm. A volume of 2 ml should be sufficient for superficial block at each level.
Needle Insertion and Injection
An 18 G graduated epidural needle is used to perform the PVB. Insertion is performed at the lateral landmark described above, in an anteroposterior direction, perpendicular to skin, in the sagittal plane. The forefinger should be placed at the 35 mm depth mark on the needle, as a guard and bony contact with the TP sought. If this is not achieved, then the needle should be withdrawn to the skin and redirected in a slightly caudal direction. If this fails, slight cranial angulation should be tried. If bone contact is still not realized, then the finger guard should be replaced at 40, 45, and 50 mm, repeating the above procedure at each depth until bone is contacted.
Once contact with bone has been made, the depth should be noted and the forefinger guard moved 10 mm distally. The needle should then be withdrawn and ‘walked off’ the TP caudally, advancing until it is 10 mm deeper than the depth of first bone contact. Cranial angulation can also be used, but this is not recommended as first choice, because pleural puncture and pneumothorax may be more likely than with the caudal approach.
If it is not possible to walk-off bone, the needle is re-inserted more caudally or cranially and the process repeated. The needle should not be advanced more than 10 mm beyond the depth of contact with the TP unless there is marked resistance to injection of the local anaesthetic agent. If this occurs, the needle should be advanced cautiously using the ‘change in resistance technique’ described below.
Together with the above procedure, paravertebral space penetration can be confirmed by the use of a syringe of saline, noting the slight change in resistance to injection as the needle is advanced beyond the costotransverse ligament. This should not be a complete loss of resistance, which, if it occurs, may indicate pleural puncture. A click may be palpable and even audible on passing through the costotransverse ligament.
Further confirmation can be sought if desired, with the use of a nerve stimulator, set at 2 Hz, with a pulse width of 0.3 ms, and current of 2 mA. Intercostal or abdominal muscle contraction should be apparent when the needle tip is in the appropriate position. This technique is particularly useful in the lower thoracic and lumbar regions.
After careful aspiration to confirm that the needle tip is not intravascular or intrathecal, the predetermined dose of local anaesthetic should be slowly administered.
Catheter Insertion for Continuous Analgesia
A catheter can be inserted into the paravertebral space for continuous postoperative analgesia. This is especially useful after major surgery and for the management of unilateral rib fractures. After the paravertebral space has been located, 5–10 ml of local anaesthetic or normal saline is injected to expand the space and a standard epidural catheter is then advanced no more than 2 cm into the space. Compared with epidural catheterization, slightly more force is required to thread a paravertebral catheter. Deeper catheter insertion increases the risk of intercostal or epidural cannulation.
Paravertebral catheters can be very reliably inserted under direct vision by the surgeon during thoracotomy. The success rate is much higher if tears and incisions in the medial parietal pleura are repaired before closure. The insertion of paravertebral catheters during video-assisted thoracoscopic surgery has also been described.
Ultrasound Guidance for PVB
Various positions for probe alignment and angles of approach for needle insertion have been suggested. The authors recommend that a linear probe set at 5 MHz is selected and placed about 5 cm from the midline in a craniocaudal direction. An anatomical survey is carried out and the ribs, posterior (internal) intercostal membrane (PIM), and pleura are all identified (Fig. 2).
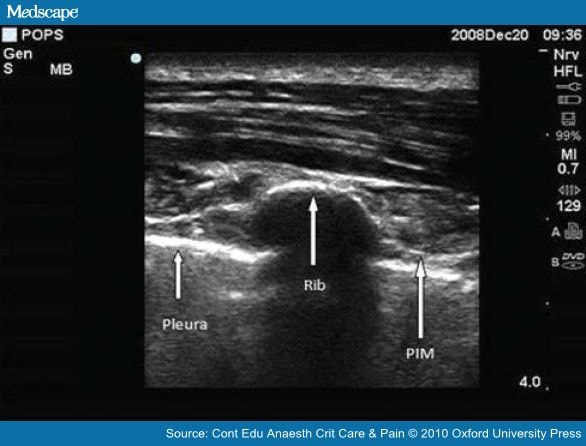
Figure 2. Ultrasound scan of the chest wall showing the rib, pleura, and PIM.
The probe is then moved medially to show the bony transition from rib to TP (Fig. 3). The TP is always more superficial than the rib.
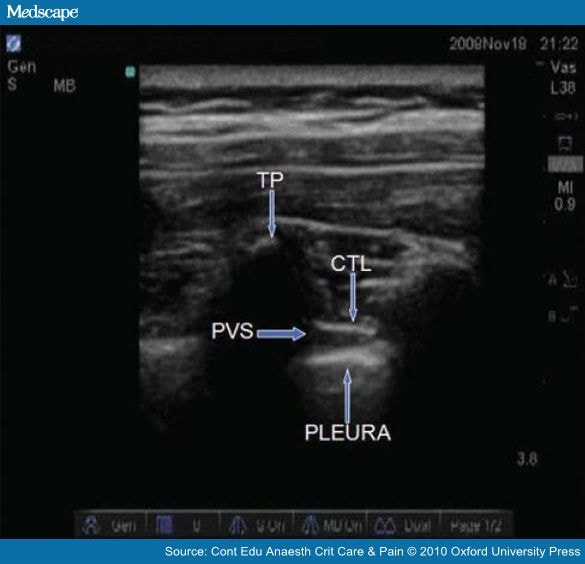
Figure 3. Ultrasound scan of the posterior chest wall showing the TP, costotransverse ligament (CTL), paravertebral space (PVS), and pleura.
The pleura will become less distinct at this point, so the probe is angulated laterally to improve the image and measure the distances between the skin, TP, and pleura. The TP can then be marked at the midpoint of the probe.
In the ultrasound-assisted approach, the probe is then removed and the block performed as previously described, using the depth information to improve needle placement. The actual needle to bone distance is usually slightly greater due to tissue compression by the probe.
In the ultrasound-guided approach, the needle is inserted into the paravertebral space alongside the probe in an ‘out-of-plane’ technique. As local anaesthetic is injected, the space between the pleura and costotransverse ligament will be seen to expand. This expansion can be followed cranially and caudally to assess the need for additional injections. A catheter can then be inserted and the position confirmed.
Testing the Block
If the patient is to be awake or sedated for surgery, anaesthesia should be assessed by the response to pinprick or ice, 10–15 min later. Additional injections can then be performed at the appropriate levels if the block is incomplete.
Volume of Injectate Needed and Pattern of Spread
Levobupivacaine and ropivacaine are the most widely used local anaesthetic agents for PVB in current practice. The use of neurolytic agents is almost exclusively restricted to patients with pain from terminal cancer, whereas steroids are sometimes administered in patients with chronic pain conditions.
There is no reliable relationship between the extent of spread and the volume of injectate. Local anaesthetic extends mainly in a craniocaudal direction, but it can also spread in the prevertebral plane, and into the epidural, intercostal spaces, or both to a variable extent (Fig. 4).
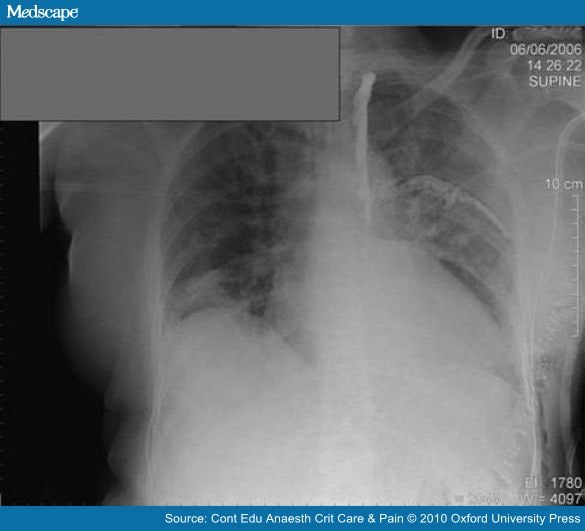
Figure 4. Chest X-ray showing spread of contrast in the paravertebral space and into an intercostal space.
Current recommendations are based on clinical experience, cadaveric, and radiographic studies.[4–6] A single injection of 15 ml of local anaesthetic produces a somatic block over a median of three dermatomes and a sympathetic block over eight dermatomes.[6]
The spread of injectate in the paravertebral space is less in women compared with men. Therefore, to ensure reliable and widespread cover, multiple injections of 3–5 ml at each thoracic vertebra are required. Another approach is to block alternate levels, or to perform injections at the upper and lower dermatomal levels. In children, a volume of 0.5 ml kg−1 covers an average of four dermatomes.[2] For continuous local anaesthetic infusions, a rate of 0.1 ml kg−1 h−1 for adults and 0.2 ml kg−1h−1 for children is recommended.[2]
The addition of opioids to the local anaesthetic infusions does not confer any benefit, but clonidine 1 µg kg−1 can improve the quality and duration of analgesia.
As with any other local anaesthetic infusion technique, the infusion rate should be titrated to effect and care must be taken to ensure that the dose does not exceed the maximum recommended.
Advantages of PVB
- PVB is easier to learn and perform than thoracic epidural anaesthesia.
- Analgesia is comparable with that provided by a thoracic epidural, in terms of success rate and analgesic efficacy.
- PVB can be performed safely in fully anaesthetized patients.
- There is less risk of neurological complications than with most other regional anaesthetic techniques.
- Pronounced hypotension is unusual because sympathetic block is rarely bilateral.[6, 7]
- Urinary retention does not occur, unlike neuraxial techniques.
- There is less sedation, nausea, vomiting, and constipation compared with opioid-based analgesic techniques, as opioid consumption is considerably reduced. Enteral nutrition and mobilization should therefore be achieved earlier.
- Compared with interpleural blocks, PVB analgesia is more intense and longer lasting. Serum levels of local anaesthetic are lower.
- PVBs have been shown to reduce chronic pain after thoracic and breast surgery.[8, 9] This is possibly because of intense block of both the sympathetic and somatic nerves, preventing sensitization of the central nervous system andN-methyl-D-aspartate receptor ‘wind up’.
- Tumour recurrence after breast surgery may also be inhibited.[10]
- Less perioperative morbidity and a shorter hospital stay could potentially result in cost savings, but this is unproven.
Complications
The overall incidence of reported complications with PVBs is between 2.6% and 5%;[4, 7] however, the risk of long-term morbidity is exceedingly low. No fatality directly attributable to PVBs has been reported.[2] The failure rate in experienced hands varies between 6.8% and 10%,[4, 7] which is broadly comparable with epidural analgesia. Other specifically reported complications include: hypotension 4.6%, vascular puncture 3.8%, pleural puncture 1.1%, and pneumothorax 0.5%.[4]
Inadvertent pleural puncture may not be recognized, as a short but effective interpleural block will result. The actual frequency of this complication may therefore exceed 1.1%, particularly with the cranial approach. If pleural puncture is appreciated, an interpleural block can be performed intentionally and a catheter inserted to prolong analgesia. Pneumothorax only rarely follows pleural puncture but when it occurs, it is usually small and can therefore be managed conservatively.[7] Tension pneumothorax is a potential complication in ventilated patients, but no cases have as yet been reported.
Bilateral block has been reported in up to 10% of cases, which is usually due to epidural spread and less commonly to mass movement of the drug across the midline in the prevertebral plane. Epidural spread is more common with a more medial injection site and with catheter techniques, although block distribution tends to be less on the contralateral side.
Ipsilateral Horner’s syndrome is a common side-effect with blocks extending to T1 and T2. Total spinal anaesthesia is very rare and has only been reported twice in the world literature. However, if the plane of approach of the needle is close to the midline, the dural cuff surrounding the intercostal nerve can be penetrated.[2]
Conclusion
PVBs are easy to perform, have a high success rate, and offer significant potential advantages to patients, compared with other regional techniques and opioid-based analgesia. PVBs are associated with a low rate of complications. The likelihood of significant long-term morbidity is low.
PVBs should be strongly considered for all unilateral surgery in the thoracic or abdominal region as an alternative to thoracic epidural or systemic opiate analgesia.
Sidebar
Key Points
- Paravertebral blocks (PVBs) are easy to learn and perform.
- They are a very effective way of providing analgesia for unilateral surgical procedures or painful conditions of the thorax and abdomen.
- The success rate and analgesic efficacy of PVBs are comparable with epidural block.
- When performed correctly, complication rates are low.
References
- Richardson J, Lonnqvist PA. Thoracic paravertebral block (review article). Br J Anaesth 1988;81:230–8.
- Karmakar MK. Thoracic paravertebral block (review article). Anesthesiology 2001;95:771–80.
- Eason MJ, Wyatt R. Paravertebral thoracic block—a reappraisal. Anaesthesia 1979;34:638–42.
- Coveney E, Weltz CR, Greengrass R, et al. Use of paravertebral block anaesthesia in the surgical management of breast cancer: experience in 156 cases. Ann Surg 1998;227:496–501.
- Conacher ID, Kokri M. Postoperative paravertebral blocks for thoracic surgery: a radiological appraisal. Br J Anaesth1987;59:155–61.
- Cheema SPS, Ilsley D, Richardson J, Sabanathan S. A thermographic study of paravertebral analgesia. Anaesthesia1995;50:118–21.
- Lonnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade: failure rate and complications.Anaesthesia 1995;50:813–5.
- Kairaluoma P, et al. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery.Anesth Analg 2006;103:703–8.
- Richardson J, Sabanathan S, Meams AJ, Sides C, Goulden CP. Post-thoracotomy neuralgia. Pain Clin 1994;7:87–97.
- Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology 2006;105:660–4.
- Lönnqvist PA, Richardson J. Use of paravertebral blockade in children. Tech Reg Anesth Pain Manage 1999;3:184–8.
Tags: Anestesia




One Response to “Paravertebral Block”
Trackbacks/Pingbacks
Leave a Reply
You must be logged in to post a comment.