Paul M. Fenton, FFARCSI, DTM & H
Transfusion Alter Transfusion Med. 2008;10(2):82-89. ©2008 Blackwell Publishing – Posted 12/02/2008
Death from acute blood loss is a common event in many parts of the world. Most of the half-million-plus maternal deaths worldwide are due to postpartum hemorrhage. While a state of general nonreportage exists in under-resourced countries, it is clear that shortage of blood and trained clinical manpower are important causes of mortality, even for those lucky few who do make it to hospital. These factors and methods of resuscitation are considered in this review of the clinical scene and simple techniques available to treat hemorrhagic shock in under-resourced countries.
Background: The Problem in Poor Countries
The occurrence of death from acute blood loss is as common today as it has been at any time. Hemorrhage during childbirth and after trauma from road traffic accidents and assaults are the leading causes, with deaths taking place either within poorly resourced health facilities or elsewhere – on the road, in a village or in an urban slum without transport or expert help.
The rate of cesarean delivery is only about 1% of all births but, even so, obstetric surgical output in Africa far exceeds that from general surgery.[1] The author has visited many hospitals in rural areas where every major operation performed is either an emergency cesarean section or a laparotomy for obstetric-related abdominal sepsis, both frequently complicated by life-threatening anemia. However, most of the estimated half-million-plus maternal deaths worldwide are due to hemorrhagic hypovolemic shock occurring outside a health facility.
While concern since 1985 about increasing maternal mortality rates[2] has not reduced maternal deaths in Africa, it has at least provided the stimulus to gather data. These hardly exist at all in the sector of general surgery, the practice of which has virtually ceased to exist in much of rural Africa. It was recently observed that ‘surgery [and by association, anesthesia] has failed the people of Africa’.[3] The preponderance of obstetric hemorrhage and the dearth of general surgical data has therefore given this review an obstetric skew in a mainly African setting. The author believes that the priorities lie in this direction.
Since the mid-1980s, accelerating through the 1990s, a decline in the standards of care provided by publicly funded hospitals has occurred simultaneously with the global phenomenon of urban drift and the ascendancy of global market forces. The present 1 billion slum-dweller population is projected to rise to 3 billion by 2050, all of whom will depend upon a dysfunctional public health sector.[4]
In 2007, with ‘managed health care’ still spreading from North to South, there have been management plans, strengthening of health-care delivery, strategies for health, practice guidelines, etc., but few people left in a hands-on clinical role to carry them out. Clinicians – whether nationals of poor countries or outsiders – who once would have made a career in developing the health services of under-resourced countries have gone elsewhere for a living. It has been estimated that a worldwide shortfall of 4.3 million health-care workers exists, mostly in poor countries.[5]
In 2005 a 1200-bed state hospital in one African country with 12 million inhabitants had a budget of $0.68 per patient per day, approximately 1/2000 that of Southampton General Hospital, UK, which spent $1300 per patient per day (Medical Director, Southampton General Hospital, 2005, personal communication). Qualitatively, such a funding differential defies comprehension – it conveys only the near impossibility of adequate service provision and the critical examination that must attend any new development initiative if it is to bring benefit.
It is not all bad news, however. Properly allocated health development funds can bring results. The author recently visited Malawi, Africa, where a new European Union-funded blood transfusion service has greatly increased the safety and availability of blood. The previously common occurrence of death from anemia on the operating table is now a rarity in that country.
Likewise, the new ‘sector-wide approach’ recently adopted by aid donors has enabled funding of actual health services and is showing results. It remains to be seen whether these services can make up for the wastage of health aid expenditure that has occurred over the preceding two decades.
The Need for Blood Transfusion
The ultimate sole treatment to save life in cases of acute blood loss is to transfuse blood. Recent advances in oxygen-carrying hemoglobin (Hb) substitutes are not relevant in the context of limited resource settings, and these artificial oxygen carriers have yet to be approved for clinical use in the USA and Europe. Usually only one, at most two, units of blood are available per patient. Other blood products such as fresh-frozen plasma, cryoprecipitate and platelet concentrates are unavailable. Preoperative elective measures such as autologous predonation are usually not feasible.
Faced with continuing heavier than expected blood loss, but having no blood to give, the clinician is obliged first to estimate, then to optimize, an invisible balance between blood loss, blood pressure and the correct infusion rate and type of clear intravenous fluid to give. He or she is otherwise reduced to an impotent observer of increasing pallor and eventual asystole in a patient who, most likely, has a healthy cardiovascular system.
Pre-existing Anemia
The ‘reference Hb’ range for a particular population differs around the globe, as it takes into account disorders that reduce Hb, and no absolute figure for Hb or iron stores (ferritin) exists, below which a person may be called ‘anemic’. However, the World Health Organization defines anemia in pregnancy as Hb < 11 g/dL.[6] Increasing age also causes a slight fall in Hb.[7] It is not known whether a chronically low Hb confers any compensating mechanisms that may affect oxygen delivery to vital organs and improve survival during acute blood loss. In other words, will a starting Hb of 11 g/dL give a different outcome, compared with 14 g/dL, in the event of a 3 L blood loss resulting in a final Hb of 4 g/dL?
There is no evidence, despite publications and community initiatives over two decades, that mild to moderate anemia has a significant effect on maternal mortality. An average anemia as low as 4-8 g/dL (defined in one study as ‘moderate’) was shown to give only a 1.35-fold increase in overall maternal mortality from all causes.[8]
Human evolution has adapted us to survive considerable blood loss. The oxygen-carrying capacity of blood is some three to four times that required to satisfy minimum oxygen consumption, although such overcapacity has other functions also. The whole-body adult oxygen consumption at rest of 250 mL/minute declines when the Hb level falls below 4 g/dL. In terms of oxygen delivery, 4 g/dL may therefore be regarded as the lowest figure for an absolute state of anemia, compatible with survival only when all other systems are functioning optimally. However, supply-dependent conditions of oxygen consumption in splanchnic vascular beds have been shown to occur at higher levels of Hb during elective surgery.[9]
During late pregnancy, the increased circulating volume, dilutional anemia and increases in clotting tendency are other evolutionary effects that mitigate the lethal results of blood loss.
Recognizing Situations of Acute Hemorrhage
Even in developed countries, it is common for case histories of death from excessive blood loss to be complicated by problems of appropriate staff being unavailable or off-duty, poor communication between junior and senior medical staff, and panicky treatment administered haphazardly with inadequate or malfunctioning equipment. These factors have an especially significant impact in poorly resourced hospitals. In addition, in the instance of concealed hemorrhage, there is often a diagnostic delay on admission or delay in action during the postoperative period. In the author’s experience, by far the commonest time of death for all patients is during the postoperative period because of hemorrhage and inadequate intravenous fluids.
Patients admitted for resuscitation while overtly bleeding or who suffer unexpected intraoperative hemorrhage present a clearer direction for the clinician. Early intervention is as important as the subsequent management. Concealed bleeding from a ruptured uterus or fractured pelvis is often missed or underestimated. The clinician should look for the signs of tachypnea, thirst, tachycardia, hypotension, cold extremities, reduced urine output and decreased conscious level. Simple clinical assessment of these signs of circulating volume status has been shown to be as effective as invasive monitoring when used for preoperative fluid optimization after femoral fracture.[10]
In obstetrics, the incidence of, and mortality from, postpartum hemorrhage (PPH) is much greater than from antepartum hemorrhage.[11] The clinical duty of effective, repeated follow-up cannot be overemphasized.
The Basic Support Essentials
The reader is referred to The Clinical Use of Blood and Surgical Care at the District Hospital[12] for more detailed information on intravenous fluids and resuscitation.
It is fortunate for the clinician working with limited resources that, with the exception of insufficient blood for transfusion, he or she can achieve results on a par with the most sophisticated, well-equipped medical institutions in the West. Apart from improved diagnostics, such as from CT scanning or ultrasound, the basics of resuscitation methods and equipment are cheap, standardized and available worldwide, in any hospital large or small. Even a laboratory-determined Hb can be replaced by examination of the nail beds, palms, conjunctivae and tongue (Figure 1).
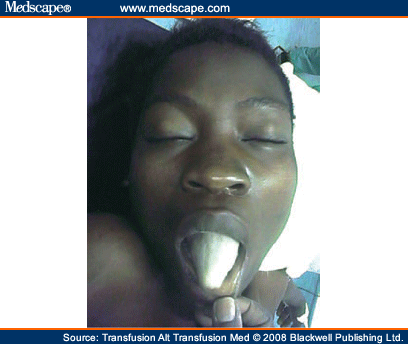
Figure 1.Severe anemia from ruptured uterus.
The simple measures are well known and do not need laborious repetition here: head down, legs up position, oxygen, warming blanket, urinary catheter, warm intravenous fluids and use of pressure bags for rapid infusion.
There should be two 14-gauge intravenous cannulae in place, avoiding veins near joints, of which one should be in the R internal jugular vein (IJV). (See Surgical Care at the District Hospital[12] for placement.)
Immediate Surgery
No severely bleeding patient has a chance unless the surgeon can get in to secure hemostasis, preferably with anesthesia. No rule can be laid down about the right preoperative blood pressure, but common sense dictates that if the intravascular volume balance between infusion and blood loss is tipping the wrong way and a treatable diagnosis has been made, surgery must begin no matter what the cardiovascular status.
Surgical Measures to Reduce Blood Loss
Packing, continuous direct pressure and traction on packs are all techniques to stop bleeding, often left in situ until the next day while the patient is kept sedated and ventilated overnight in a high dependency area – an essential component of care after major surgery when packs are left in situ.
The atonic uterus is the commonest obstetrical problem. After rubbing up the fundus, giving intravenous oxytocin and ergometrine, intrauterine injection of oxytocics may be used. Traditional surgical techniques have included ligation of the uterine or internal iliac arteries, and hysterectomy. The simpler technique of B-Lynch suture has been described.[13] It requires a laparotomy, but avoids hysterectomy. Also, if available, a balloon can be inflated in the uterus.
Intraoperative blood salvage can be carried out even if dedicated disposable apparatus is not available. Blood collected in a galipot is filtered through gauze swabs and then aspirated into a standard blood bag containing anticoagulant by siphoning, and given back to the patient through a filtered giving set. Clotting factors are probably absent. However, care should be taken with the diagnosis: if there is any suspicion of sepsis, e.g. from a ruptured ectopic pregnancy coinciding with pelvic sepsis or any contamination of blood with bowel contents or pancreatic fluid, or any odor from the blood, it should not be transfused. Rapid onset of endotoxemia and cardiac standstill can result from transfusion of contaminated blood.
More detailed surgical management of PPH with minimal resources has been published.[14]
Infusion Fluids
Resuscitation fluids in common use are crystalloids: normal saline and Ringer’s lactate (Hartmann’s), and colloids: gelatins, dextrans and hydroxyethyl starch (HES). Locally manufactured fluids in bottles present a problem for rapid pressurized transfusion: air should not be pumped into the bottle as the risk of fatal massive air embolus is high when the transfusion finishes.
It is usual to give three times the estimated blood loss as crystalloid. This rule has to be loosely implemented because the losses are not known with certainty and the supplies of second-line fluids, colloids and, ultimately, blood may be limited. For adults, after 3-5 L of crystalloid and 1.5 L of colloid, there should be some surgical control and a blood transfusion should be imminent. Otherwise, the prognosis looks poor.
More important than circulating Hb is circulating volume. No controlled trial has ever been carried out, for obvious ethical reasons, but it has been shown that shocked patients can suffer massive blood loss, receive little or no transfused blood yet still have a favorable outcome despite severe anemia, provided circulating volume is maintained.[15,16] Furthermore, the promise of blood may actually worsen outcome if clear fluid infusions are slowed during blood transfusion or while ‘waiting for blood’.
The patient’s response to infusion should be carefully observed: the blood pressure rises (or becomes recordable) and the jugular venous pressure (JVP) becomes visible. It is easy to approximately measure the JVP using a simple manometer attached via a three-way tap to the IJV line. Any value above zero measured at the midaxillary line shows improvement of volume status, and a reading of > 5 cm H2O means infusions may be slowed.
The physiological response to hypovolemia is vasoconstriction, which diverts blood to the central circulation. The extent of this life-saving response depends upon the rapidity of the initial hemorrhage and the time over which vasoconstriction has developed, as well as other factors such as sepsis, malaria or anesthesia, which all tend to cause vasodilatation. The offset of vasoconstriction is unpredictable with considerable variation between individuals and probably between races also. The practical implication of this is that some patients may more easily be overtransfused (with resultant pulmonary edema and/or acute cardiac dilatation) when in a vasoconstricted state during the early stages of shock resuscitation, especially when severe dilutional anemia coexists. During rapid infusions, it is important, therefore, constantly to observe the response of central venous and arterial pressures and moderate the rate of fluid infusion accordingly.
Colloids are normally given on a one-to-one basis with blood loss. While the dextrans do interfere with blood crossmatching, colloids have not been clinically proven to worsen bleeding in a hemorrhaging patient. The clinical suspicion that colloids cause bleeding may have arisen because, being scarce, they are administered too late and thus associated with irretrievable hemorrhage.
The effectiveness of a single dose of the antifibrinolytic drug tranexamic acid is currently being investigated in the CRASH-2 trial.[17] Even if proven to be cheap and effective, the therapy will not necessarily become available in countries where procurement systems are generally collapsed and clinicians have little input to government decision-making on expenditure.
Effects of Hemodilution with Crystalloid and Colloid
There is evidence that hemodilution of blood results in acceleration of coagulation tested in vitro, depending on the nature of the diluting fluid.[18-20]
Dilution with crystalloids accelerates coagulation up to 40% dilution and does not cause any coagulopathy until at least 60% dilution. Dilution with various colloids, however, impairs coagulation to varying degrees depending on the colloid. The high-molecular-weight colloids, particularly high-molecular-weight HES, impair coagulation at approximately 20% dilution, whereas the gelatins, albumin and the newer HES products (particularly HES 130/0.4) do not impair coagulation until 40% dilution or more.[21]
Studies have suggested that saline may impair coagulation to a greater degree than Ringer’s lactate but also that there is no difference between the two.[22] The effect of diluting calcium with calcium-free fluids may be important. Recently, Kiraly et al.[23] compared the bleeding from liver laceration in pigs when resuscitated with either saline or Ringer’s lactate. The saline group demonstrated significantly greater coagulopathy and hemorrhage than that given Ringer’s lactate. There is very recent evidence that HES suspended in saline interferes more with coagulation than HES suspended in a balanced salt solution.[24]
The event of blood loss itself in volunteers without any intravenous infusion has been shown by thromboelastography to increase coagulation, an effect only partly explained by autohemodilution.[25] In addition, there appears to be a clinically important acute traumatic coagulopathy, also unrelated to fluid administration.[26]
However, the results of in vitro thromboelastography, the carefully controlled normovolemic measurements made in vivo, and the hypovolemic animal model should be applied with caution to real-life resuscitation situations in acute hemorrhagic shock where other conditions, such as extensive tissue damage, disseminated intravascular coagulation, toxemia, sepsis and malaria, may be concurrent with anesthesia administration. The enhanced clotting effect is in any case transient with the risk of rebleeding in the immediate postoperative period.[27]
Apart from the coagulation effects, saline loads may be less well cleared through the kidney and may result in greater tissue edema in the postoperative period, but there are no clinical studies to support an outcome deficit.
In summary, crystalloids increase the clotting tendency, but during massive infusions, saline and saline-based colloids are more likely to produce coagulation abnormalities than balanced salt solutions, perhaps by diluting calcium or by some more complex mechanism not yet fully understood. However, Ringer’s lactate is hypo-osmolar and this may be harmful in patients with head injury. A simple approach would be to use Ringer’s lactate as the basic resuscitation solution for acute hemorrhage, but when there is concurrent head injury, use alternate bags of saline and Ringer’s lactate to keep the plasma osmolarity closer to normal.
When to Give Blood
Transfusion of precious bags/units of whole blood should obviously be delayed until the optimal moment. Only clinical judgment, decided individually for each case, can determine when this point has been reached. Problems of low blood pressure and reduced circulating volume should ideally be treated with crystalloid and colloid during which time hemostasis is attained. Therefore, if it is possible, blood transfusion should not form part of resuscitation, but be given after control is achieved.
The situation of giving blood while a patient is still actively hemorrhaging should be avoided at all costs – the blood will be wasted, subsequent anemia will be untreatable, and it means, in any case, that the situation has probably passed beyond control.
The aim of blood transfusion should be to treat life-threatening anemia after the hemorrhagic event. Even one unit can make the difference between life and death as each unit should raise the Hb level by approximately 1.5 g/dL and can tip the balance toward compensation, rather than decompensation, in a critically ill patient.[28]
Anesthesia
Techniques of anesthesia should aim to reduce bleeding, maintain blood pressure, improve oxygen delivery and optimize postoperative outcome. Simplicity and vigilance are more important than sophisticated drugs and techniques.
The recommended, safe, low-cost drugs for induction of anesthesia are intravenous ketamine (1 mg/kg) and suxamethonium (1 mg/kg) given into the IJV line, combined with a rapid-sequence intubation technique. Blood pressure may fall after a muscle relaxant relaxes a tense abdomen that is splinting a site of hemorrhage.
Further doses of ketamine or preferably 2-3% ether may used for maintenance, increasing dose as the condition improves, using the maximum available oxygen concentration for lung ventilation. Ether and ketamine are life-preserving drugs in all cases of shock requiring surgery, and both cause uterine contraction. Halothane is to be avoided in obstetric bleeding because of uterine relaxation and myocardial depression, and it has caused postoperative hemorrhage and death even after an uncomplicated cesarean section.[29] Breathing may be spontaneous or controlled, although hypercarbia and deep planes of anesthesia should be avoided.
Once surgical control of bleeding is assured, blood pressure should be kept at low to normal levels, and opiates should be used postoperatively to avoid reactive hemorrhage caused by postoperative pain.
Continuing Hemorrhage
Disseminated intravascular coagulation (DIC) may be considered, although little can be done apart from the measures outlined above. Blood in a small glass tube, held within a closed hand to simulate 37°C, will fail to clot even after 15-20 minutes. DIC ideally requires fresh-frozen plasma or preferably cryoprecipitate, which is rich in factor VIII and fibrinogen.
Postoperative Hemorrhage
The gulf between the usefulness of what is written from an evidence base and what is obviously simple, good, clinical practice is best illustrated in the postoperative period: no study has shown that any particular treatment improves outcome in primary or secondary PPH.[30,31] However, the finding of a severely shocked or dead patient lying in a pool of blood a few hours after surgery is so common that it is mandatory to check every major case, obstetric and general surgical – several times – in the postoperative period, assess volume status, ensure adequacy of intravenous infusion and institute proactive measures, such as a return to the operating theater for investigation of bleeding (Figures 2, 3).
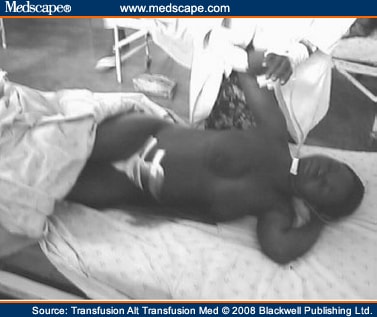
Figure 2.A common problem: post-cesarean section hemorrhage.
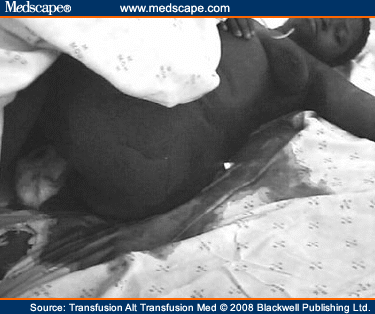
Figure 3.Urgent return to operating theater needed.
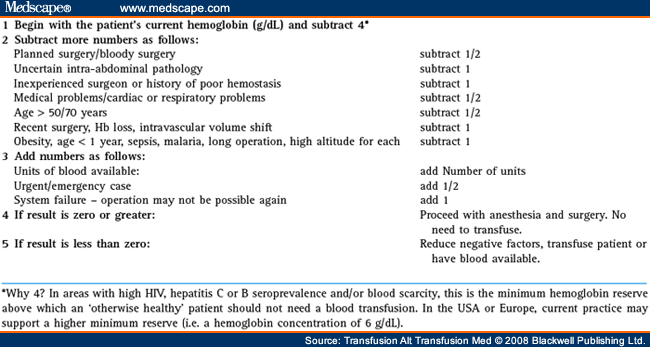
An Algorithm for Determining the Necessity for Transfusion[32]
The algorithm described in Table 1 has proved a useful guide and training tool in deciding when to give scarce blood of uncertain safety in the pre- and postoperative periods
Conclusion
Successful resuscitation of the acutely hemorrhaging patient remains an attainable goal in resource-poor settings. Sophisticated or high technology management offers only marginal advantages over simple low-cost methods which have the added advantage of being universally familiar. It is clear that, providing circulating volume is maintained, principally with infusions of crystalloid, blood transfusion can safely be reserved for treatment of life-threatening anemia rather than hemorrhagic shock and the patient outcome can be optimized.
However, the downward trends in the provision of government clinical services seen in recent decades, especially in manpower, will most likely continue or even worsen in the foreseeable future for the poorer locations. The fact of easily administered clinical remedies will therefore assume limited relevance in such a context.
CLICK HERE for subscription information about this journal.
Table 1. Begin with the Patient’s Current Hemoglobin
![]()
References
|
The author would like to thank Dr Jean Emmanuel who critically read this review and Prof. Mike James for his invaluable advice.
Correspondence to Dr P. M. Fenton, Meyra, 47800 Agnac, France. E-mail: fenton.paul@wanadoo.fr
![]()




One Response to “Managing Situations of Acute Blood Loss with Limited Resources”
Trackbacks/Pingbacks
Leave a Reply
You must be logged in to post a comment.